Research Article Open Access
A Pragmatic Evaluation of the National Cancer Institute Physician Data Query (PDQ)®-Based Brief Counseling on Cancer-Related Fatigue among Patients Undergoing Radiation Therapy
Joshua Bauml1, Sharon X Xie2, Courtney Penn BA3, Krupali Desai3, Kimberly W Dong3, Deborah Watkins Bruner1,4,5, Neha Vapiwala1,4 and Jun James Mao1,2,3*
1Abramson Cancer Center, University of Pennsylvania, 3400 Spruce Street / 2 Gates, Philadelphia, Pennsylvania 19104, USA
2Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, 3400 Spruce Street/2 Gates, Philadelphia, Pennsylvania 19104, USA
3Department of Family Medicine and Community Health, University of Pennsylvania, 3400 Spruce Street/2 Gates, Philadelphia, Pennsylvania 19104, USA
4Department of Radiation Oncology, Perelman School of Medicine, University of Pennsylvania, 3400 Spruce Street / 2 Gates, Philadelphia, Pennsylvania 19104, USA
5Woodruff School of Nursing, University of Emory, Georgia 30322, USA
- *Corresponding Author:
- Dr. Jun J Mao, MD, MSCE
Department of Family Medicine and Community Health
University of Pennsylvania, 3400 Spruce Street / 2 Gates
Philadelphia, Pennsylvania 19104, USA
Tel: 215-615-4330
Fax: 215-662-3591
E-mail: jun.mao@uphs.upenn.edu
Received date: July 13, 2012; Accepted date: August 06, 2012; Published date: August 08, 2012
Citation: Bauml J, Xie SX, Penn BAC, Desai K, Dong KW, et al. (2012) A Pragmatic Evaluation of the National Cancer Institute Physician Data Query (PDQ)®-Based Brief Counseling on Cancer-Related Fatigue among Patients Undergoing Radiation Therapy. J Palliative Care Med 2:125 doi: 10.4172/2165-7386.1000125
Copyright: © 2012 Bauml J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Purpose: Cancer-Related Fatigue (CRF) negatively affects quality of life among cancer patients. This study seeks to evaluate the outcome and patient receptiveness of a brief counseling program based on National Cancer Institute (NCI) PDQ® information to manage CRF when integrated into Radiation Therapy (RT). Methods: We conducted a prospective cohort study among patients undergoing non-palliative RT. Patients with stage I-III tumors and with Karnofsky score 60 or better were given a ten-minute behavioral counseling session during the first two weeks of RT. The Brief Fatigue Inventory (BFI) was administered at baseline/end of RT. Results: Of 93 patients enrolled, 89% found the counseling useful and practical. By the end of RT, 59% reported increased exercise, 41.6% sought nutrition counseling, 72.7% prioritized daily activities, 74.4% took daytime naps, and 70.5% talked with other cancer patients. Regarding counseling, patients who had received chemotherapy prior to RT had no change in fatigue (-0.2), those who received RT alone had mild increase in fatigue (0.7, p=0.02), and those who received concurrent chemotherapy experienced a substantial increase in fatigue (3.0 to 5.2, p=0.05). Higher baseline fatigue and receipt of chemotherapy were predictive of worsened fatigue in a multivariate model (both p<0.01). Conclusion: Our data suggests that brief behavioral counseling based on NCI guidelines is well accepted by patients showing an uptake in many activities to cope with CRF. Those who receive concurrent chemotherapy and with higher baseline fatigue are at risk for worsening fatigue despite of guideline-based therapy
Keywords
Fatigue; Radiotherapy; Adverse effects; Neoplasms; Counseling; Cohort study
Introduction
Fatigue is one of the most common and debilitating symptoms affecting cancer patients and survivors [1-4]. Cancer-Related Fatigue (CRF) is defined by the National Consortium of Cancer Centers (NCCN) as a “persistent subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning” [5]. It is a major cause of non-adherence to cancer treatment regimens [6], and higher fatigue may predict worse survival [7]. CRF is associated with decreased psychological, occupational, and social functioning [8-10], and can persist for years after treatment [11-13]. The prevalence of CRF among cancer patients during treatment ranges from 25% to 99% depending on the diagnostic criteria used to determine the condition and the sample [14].
The etiology of CRF is multi-factorial including a wide range of potential physiologic and psychologic mechanisms. Models of the inflammatory cytokine activity in response to the cancer or the treatment have been amongst most extensively studied theorized mechanisms [15-17]. Additionally, neuroendocrine-based regulatory models have been implicated in many facets of fatigue [18-21]. Furthermore, co-morbidities associated with CRF are likely to contribute to its development including anemia, cachexia, depression and sleep disorders [22]. Additionally, different treatment modalities are associated with different rates of CRF [23]. Radiation Therapy [RT] has been shown to be particularly associated with the development of CRF [24]. Due to the fairly elusive underlying pathphysiology of CRF, a variety of interventions may be needed to effectively manage this condition.
Interventions have been researched to treat CRF include exercise, pharmacologic and psychosocial interventions. Numerous medications have been evaluated, although only methylphenidate has been shown to be effective. The data supporting its utility are mixed and the clinical significance of the benefit has been variable [25]. There are also a number of non-pharmacologic interventions that have been studied. Exercise, energy conservation as well as herbal remedies have been evaluated with mixed success [26-30]. At this time, there has been no treatment that improves CRF in all settings.
Evidence is limited for psychosocial interventions in the reduction of CRF during active cancer treatment [31]. The most effective interventions were those that included individual counseling sessions specifically focusing on fatigue. Behaviorally oriented interventions including aspects such as counseling and educating the patient about fatigue & how to self-care, activity management and coping techniques have been shown to be effective [32]. In addition, Cognitive Behavioral Therapy aimed at improving sleep practices has been shown to improve fatigue scores with lasting effect at follow up in one trial [33,34]. Psychosocial interventions are promising and more research must be done to determine the optimal format for delivery of information to patients, including duration and content [31].
In recognizing the clinical importance of CRF, the National Cancer Institute (NCI) Physician Data Query (PDQ)® program synthesizes the best available emerging evidence coupled with expert input and provides information via the Internet to help patients and health care providers to manage this debilitating symptom [35]. Despite the availability of this valuable resource, no study to date has attempted to translate the NCI guidelines into practice or to evaluate the outcome in a clinical setting. This effort is critically needed to ensure knowledge generated from clinical trials can become effective strategies that improve patient care. In addition, by understanding how CRF manifests in current guideline-based management we can begin designing and testing additional interventions for specific at-risk populations to create a personalized fatigue management strategy for cancer patients. Thus, the specific aims of this study are: 1) To evaluate the progression of fatigue among a cohort of RT patients who received the NCI guideline on CRF at baseline; 2) To identify factors associated with greater fatigue at the end of RT even with the guideline-based counseling; 3) To evaluate patients’ receptiveness and adherence to specific recommendations that were highlighted by the NCI guideline.
Methods
Design
We conducted a prospective cohort study among patients who were receiving non-palliative RT at the Hospital of the University of Pennsylvania (Philadelphia, PA). Inclusion criteria included being at least 18 years of age, receiving non-palliative RT treatment, being at least 14 days post-ambulatory surgery, having a Karnofsky score of 60 or better, and having an ability to read and understand English. The study excluded those with chart-documented anemia (defined as most recent hemoglobin level of less than 8.5 (g/dl) or having ongoing treatment for anemia), a known brain tumor or brain metastasis (because neurocognitive changes caused by cancer or RT could impact the completion of self-reported outcomes), and chart documentation of a recent or ongoing diagnosis of a significant depressive episode. The study was approved by the University of Pennsylvania Institutional Review Board.
Procedures
Trained Research Assistants (RAs) screened patients’ eligibility via Electronic Medical Record (EMR) and approached eligible patients for informed consent and enrollment at the baseline week of RT. Patient Reported Outcomes (PRO) along with self-reported social demographic information were collected at baseline and the last week of RT. RAs abstracted clinical information including cancer type, stage of disease, RT duration, number of RT fractions, number of weeks undergoing RT, RT dose, medical co-morbidities and medications from EMR.
Brief Behavioral Counseling: We aimed at designing brief behavioral counseling that can be easily integrated into RT care. Counseling was created based on content described on the NCI website (last modified, May 31, 2007) [35] and lasted between five and ten minutes (Table 1). An experienced mental health counselor and a palliative care physician trained RAs on delivering the content of NCI guidelines and on optimal ways of communicating the message to patients. The RAs performed the counseling face to face during the initial week of RT. A patient handout based on NCI fatigue PDQ® for patients was given to each participant at the end of counseling. To evaluate the effectiveness of the behavioral counseling, we designed a Likert scale questionnaire to ask the participant to rate the helpfulness of the counseling experience during the final week of the RT treatment course. Response options ranged from “Not at all” to “Very Much So”. We also asked the patient to self-report use of specific activities highlighted in the guideline at this time. For specific therapies, we then asked participants to rate the helpfulness from “Not at all helpful” to “Very Helpful”.
| Try to sleep at least 8 hours each night |
| Plan time to rest/take naps |
| Try not to do too much |
| Exercise |
| Plan a work schedule that is right for you |
| Let others help you at home |
| Learn from others who have cancer |
| Plan a radiation schedule that fits you |
| Talk with your doctor or nurse |
Table 1: Interventions recommended to patients to cope with cancer-related fatigue.
Outcomes
Brief Fatigue Inventory (BFI)-Primary outcome: The BFI consists of 9 items that measure the severity and interference of fatigue. The average of the 0-10 scores of the items yield a global index score with a higher number indicating more severe fatigue. The BFI is easy to understand, has strong internal consistency reliabilities (Cronbach’s α is 0.96), correlates well with other measures of fatigue [36], and appears to be responsive over time [37] as well as to interventions [38].
MD Anderson Symptom and Interference Inventory (MDASI) – secondary outcome: The MDASI is an instrument that measures common symptoms in cancer patients undergoing treatment with demonstrated reliability and validity [39,40]. It contains 19 items that measure the severity of a variety of symptoms and their impact on daily functioning. Of the 19 items, 13 assess the severity of the following symptoms: pain, fatigue, nausea, disturbed sleep, distress, shortness of breath, memory loss, appetite loss, drowsiness, dry mouth, sadness, vomiting, and numbness /tingling. These items are graded from 0 (the symptom is “not present) to 10 (the symptom is “as bad as you can imagine”). The remaining six items measure how the symptoms impact general activity, mood, work, relations with other people, walking, and enjoyment of life. These items are also graded from 0 (the symptom “did not interfere”) to 10 (the symptom “interfered completely”). The scale has two domains: symptom severity and interference. We incorporated MDASI into this research to understand the relationship between fatigue and overall symptom distress in this population.
Data analyses
Statistical analysis was performed using STATA 10.0 for Windows (STATA Corporation, College Station, TX). Appropriate descriptive statistics including examination of proportion mean, median, and range were performed for both outcomes and relevant covariates. We then performed paired t-test for BFI and MDASI comparing before and after RT. Bivariable analyses were performed to identify any socio-demographic and clinical variables that are associated with BFI at the end of RT. We then developed multivariate regression analysis with BFI at the end of RT as the dependent variable incorporating those covariates that were significant at the 0.10 level as independent variables. Because observed differences in fatigue progression varied by the receipt of chemotherapy status, we also performed a paired t-test stratified by chemotherapy status. Further, we performed a Pearson correlation between BFI and MDASI scores to understand how fatigue relates to overall symptom severity and interference. All statistical analyses were two-sided with p<0.05 indicating significance.
Results
Characteristics of the sample
Of the 115 eligible patients based on initial screening, 109 were approached and 100 (91.7%) agreed to participate. Among the 9 (8.2%) patients that declined to participate, 6 (5.5%) did not want to participate in a research study, 2 (1.8%) were fearful of releasing personal or health information, and 1 (0.9%) did not want to consider negative side effects of treatment. Additionally, 4 subjects withdrew consent and 3 subjects discontinued because of hospitalization prior to the behavior counseling, resulting in the final sample of 93 and a response rate of 85.3%. Of the 93 subjects enrolled (Table 2), 57 (61.3%) were men, 65 (69.9%) were white, 44 (48.4%) had gone to college, 40 (44%) were employed full-time at the time of the survey, and the mean age was 65 years at the time of the survey. The most prevalent tumor type was prostate (47.3%), then breast (29%), with a mix of tumors (23.7%) for the remaining study population. A large portion of the subjects (69.9%) had not received any chemotherapy in the course of their treatment, and 17.2% received concurrent chemotherapy and during the course of radiation therapy. The average cumulative radiation dose was 7200 cGy, with a range of 4500 to 8000 cGy. Enrolled subjects generally had other comorbid conditions and were on numerous medications.
| Demographic | ||
| Age (Median, range) | 65 | 38-85 |
| Sex (N, %) | ||
| Male | 57 | 61.3 |
| Female | 36 | 38.7 |
| Race/Ethnicity (N, %) | ||
| White | 65 | 69.9 |
| Non-white | 28 | 30.1 |
| Education (N, %) | ||
| Graduate or professional school | 24 | 26.4 |
| College or some college | 44 | 48.4 |
| High school or less | 23 | 25.3 |
| Employment (N, %) | ||
| Not Currently | 51 | 56.0 |
| Working | 40 | 44.0 |
| Clinical | ||
| Stage (N, %) | ||
| I | 42 | 44.2 |
| II | 28 | 30.4 |
| III | 23 | 24.7 |
| Diagnosis (N, %) | ||
| Prostate | 44 | 47.3 |
| Breast | 27 | 29.0 |
| Others | 22 | 23.7 |
| Surgery (N, %) | ||
| None | 48 | 51.6 |
| Yes | 45 | 48.4 |
| Chemotherapy (N, %) | ||
| None | 65 | 69.9 |
| Before | 12 | 12.9 |
| Concurrent | 16 | 17.2 |
| Hormone therapy (N, %) | ||
| None | 63 | 67.7 |
| Yes | 30 | 32.3 |
| Co-morbidities (Mean, SD) | 3.0 | 2.4 |
| Medications (Mean, SD) | 6.3 | 3.7 |
| Radiation Therapy (Median, Range) | 7200 | (4500, 8000) |
Table 2: Participant characteristics (N=93).
Progression and risk factors for fatigue
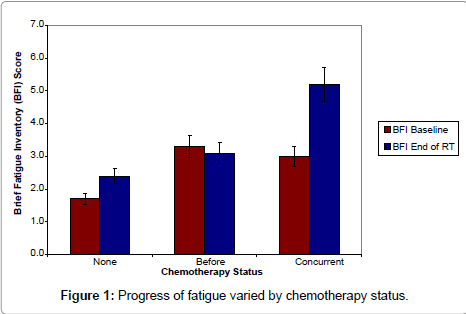
For the entire cohort, fatigue as measured by the BFI increased from 2.1 at the baseline to 2.9 at the end of RT, p<0.001. However, when stratified by chemotherapy status (Figure 1), fatigue showed a significant but small increase for those who received RT only (from 1.7 to 2.4, p=0.02) and was stable for those who received chemotherapy prior to RT (from 3.3 to 3.1, p=0.75). Nevertheless, fatigue increased substantially for those who received chemotherapy during RT, from 3.0 to 5.2, p = 0.05.
In bivariable analyses, only the baseline BFI score and chemotherapy status were associated with increased fatigue at the end of RT. In a model adjusting for these two variables, any 1 point of increase in BFI score at baseline was associated with a 0.6 point increase in BFI at the end of RT, 95% confidence interval (CI), 0.3-0.8, p<0.001. Compared with those who received RT only, individuals who had concurrent chemotherapy had 2.1 points higher in BFI score, 95% CI 0.6-3.5, p = 0.006 at the end of RT.
Fatigue and overall symptom severity and interference
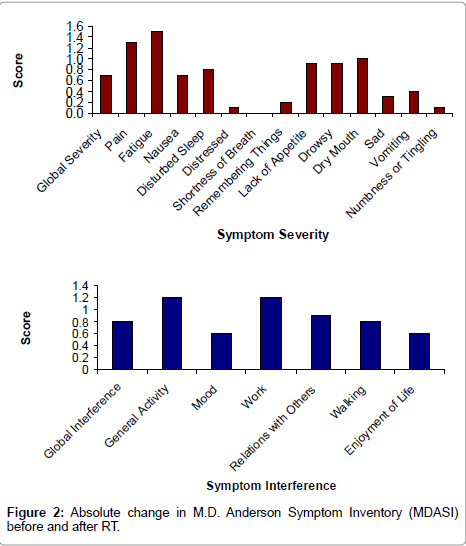
The global symptom severity and interference measured by MDASI increased significantly during the RT course, (1.2 to 1.9 for severity, p = 0.0003) and (1.4 to 2.2 for interference, p = 0.0001), (Figure 2). The fatigue item in the MDASI was also the one symptom that had the highest severity score both at baseline and end of RT and represented the most change through RT (2.1 to 3.6, p< 0.0001). The change in BFI score was highly correlated with the change in MDASI symptom severity (0.70, p< 0.0001) and symptom interference (0.81, p< 0.0001).
Evaluation of behavior counseling
The recommendations for CRF by the NCI were very well received by participants: 89% of patients found the guidelines to be useful and practical, 98% thought the material was presented by the counselor at an appropriate level, 98% could easily comprehend the material, 91% viewed the handouts (based on NCI PDQ® for patients) provided by the counselor as beneficial to their understanding, and 96% believed their questions were answered satisfactorily by the counselor.
Uptake and perceived helpfulness of activities dealing with CRF
Of the 79 available participants at the end of RT, the most common activities performed by individuals were napping during the day (74%), followed by prioritizing daily activities (73%), talking to others with cancer (71%), increasing exercise (59%), and receiving nutritional counseling (42%). While not part of NCI guidelines and not reviewed by our counselor, 33% of individuals decreased exercise and 22% took herbal or natural supplements; however, only 20% of individuals took medications.
The interventions endorsed as most helpful (“very helpful”) by individuals who used them were talking with others who had cancer (29.1%), prioritizing daily activities (28.6%), nutritional counseling (28.1%), napping during the day (26.7%), and taking medications (26.7%). The majority of patients viewed increasing exercise as at least somewhat helpful (87%), while a large proportion (61.5%) of those who decreased exercise did not believe it helped CRF at all. Interestingly, the two activities that were not endorsed by NCI - decreasing exercise (61.5%) and herbal supplements (29.4%) - had the highest proportion of individuals who perceived these activities as not helpful. Among those who took a medication (N =15), all used methylphenidate and perceived some degree of benefit from taking medication. Of the 79 available participants at the end of RT, the most common activities performed by individuals were napping during the day (74%), followed by prioritizing daily activities (73%), talking to others with cancer (71%), increasing exercise (59%), and receiving nutritional counseling (42%). While not part of NCI guidelines and not reviewed by our counselor, 33% of individuals decreased exercise and 22% took herbal or natural supplements; however, only 20% of individuals took medications.
The interventions endorsed as most helpful (“very helpful”) by individuals who used them were talking with others who had cancer (29.1%), prioritizing daily activities (28.6%), nutritional counseling (28.1%), napping during the day (26.7%), and taking medications (26.7%). The majority of patients viewed increasing exercise as at least somewhat helpful (87%), while a large proportion (61.5%) of those who decreased exercise did not believe it helped CRF at all. Interestingly, the two activities that were not endorsed by NCI - decreasing exercise (61.5%) and herbal supplements (29.4%) -had the highest proportion of individuals who perceived these activities as not helpful. Among those who took a medication (N=15), all used methylphenidate and perceived some degree of benefit from taking medication.
Discussion
This study sought to translate the NCI (PDQ)® guidelines for CRF into clinical practice and to evaluate their impact on controlling CRF. We found that a brief 5-10 minute counseling session provided by individuals without formal medical or counseling background was well-received by patients. Fatigue remained fairly stable for those who did not receive concurrent chemotherapy. Higher baseline fatigue and receipt of chemotherapy were risk factors for developing worsened fatigue at the end of RT. Fatigue was the most distressful symptom reported by participants and was highly correlated with overall symptom severity and interference on daily living. Many patients performed the activities highlighted in the guidelines and perceived them as helpful. Despite this, those who had concurrent chemotherapy experienced a substantial increase in fatigue.
It is important to acknowledge several limitations prior to discussing the implication of our findings. Our study was not a trial. It was pragmatic in nature and the lack of a control arm could not provide direct causal evidence for the specific efficacy of brief behavioral counseling for CRF. Our sample size was small, which may limit the degree to which we were able to study other prognostic factors related to CRF. Furthermore, the loss to follow-up may also affect results in unknown directions. Lastly, our study was performed in an urban tertiary cancer center, which limits the generalizability of the study.
Numerous studies have demonstrated that CRF increases over the course of RT [41-44]. Our study found [45,46] that those who did not have chemotherapy had fatigue in the “mild” range before and after RT [47] with minimal change. It is also possible that the lack of change may be due to a “response shift,” which occurs when patients become accustomed to their fatigue [48-51]. However, in contrast to a marked increase in fatigue by those who received concurrent chemotherapy, the lack of change cannot be entirely attributed to this phenomenon.
The finding that fatigue increased substantially (from 3 to 5.2 on BFI) in those who are receiving both RT and chemotherapy is consistent with previous studies that show that patients receiving a combination of the two therapies have higher levels of fatigue compared to those receiving a single therapy [23,52,53]. The CRF associated with each cancer subtype and its therapy may be distinct in its pathophysiology as well as appropriate treatments. For example, exercise is a common intervention recommendation for CRF, and an optimal regimen was found to involve moderate-intensity and resistance exercises. However, exercise has shown to be most beneficial only for breast and prostate cancer survivors. In contrast, exercise has not shown significant improvements in fatigue for leukemia, lymphoma and colorectal cancer survivors [27]. Thus interventions need to be developed and tested in the context of existing guideline-based counseling to find strategies to decrease CRF in each specific population. According to our analysis, the population of cancer patients who get concurrent radiation and chemotherapy would be a group at particularly high risk.
The vast majority of participants felt that the counseling provided helpful information in an appropriate format. As NCI PDQ® is readily available and updated regularly to incorporate research evidence and expert opinions, efforts like our study are needed to make sure that this work that can be easily incorporated into clinical care and, ultimately, affect patient care. The fact that most participants adhered to at least one of the recommended NCI interventions (Table 3) and perceived all of the interventions as at least “somewhat helpful” further suggests that our educational model could be effective in CRF management. Our high rate of participation indicates that cancer patients readily welcome suggestions on how to manage their CRF. Moreover, our intervention was administered by trained RAs. This raises the possibility that a doctor or specialist may not be required to provide this guidance. It is thus possible that a health care provider such as a medical assistant who becomes well-versed in the NCI guidelines may deliver the intervention. This can help circumvent the lack of resources and funding, which create barriers for patients and their families to utilize effective therapies [54].
| Among those who performed the interventions, perceived helpfulness of each intervention | |||||
|---|---|---|---|---|---|
| Interventions | Performed Behavior | Not at All Helpful | Somewhat Helpful | Moderately Helpful | Very Helpful |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Increase Exercise | 46 (59.0) | 6 (13.0) | 19 (41.3) | 13 (28.3) | 8 (17.4) |
| Decrease Exercise* | 26 (33.3) | 16 (61.5) | 3 (11.5) | 4 (15.4) | 3 (11.5) |
| Nutritional Counseling | 32 (41.6) | 2 (6.3) | 13 (40.6) | 8 (25.0) | 9 (28.1) |
| Prioritize Daily Activities | 56 (72.7) | 2 (3.6) | 27 (48.2) | 11 (19.6) | 16 (28.6) |
| Nap During the Day | 58 (74.4) | 3 (5.2) | 18 (31.0) | 21 (36.2) | 16 (27.6) |
| Relaxation Techniques | 21 (27.3) | 4 (19.1) | 9 (42.9) | 7 (33.3) | 1 (4.76) |
| Medication | 15 (19.5) | 0 (0.0) | 6 (40.0) | 5 (33.3) | 4 (26.7) |
| Herb/Natural Supplement* | 17 (22.1) | 5 (29.4) | 5 (29.4) | 3 (17.7) | 4 (23.5) |
| Talk to Others with Cancer | 55 (70.5) | 1 (1.8) | 30 (54.6) | 8 (14.6) | 16 (29.1) |
Table 3: Activities performed to cope with cancer-related fatigue (N=79).
To our knowledge, this is the first study to demonstrate that brief behavioral counseling based on the NCI (PDQ)® guidelines can be easily implemented in an RT setting and that it is welcomed by most cancer patients. Many patients participated in the activities recommended by the guideline and perceived them as helpful. Our study was also able to identify two high-risk groups for the development of more severe CRF: those patients who received concurrent chemotherapy and those with a higher baseline fatigue. More research is needed to develop and test innovative or tailored interventions to target these at-risk groups. With risk assessment and specific effective interventions, we can ultimately adopt a personalized fatigue management strategy for diverse populations of cancer patients.
Acknowledgements
Dr. Mao is supported by American Cancer Society (ACS) CCCDA-08-107-02 and National Institutes of Health [K23 AT004112-05]. The funding agencies had no role in the design and conduct of this study. We would like to thank Donna Pucci, James Wolf, Tam Nguyen, Ellie Barr, and Shawn Fernandez for their contribution to data collection and management. We would like to thank the patients, radiation oncologists, and staff for their support of our study. This research was presented in part as a podium presentation at the Society for Integrative Oncology Annual Meeting in New York City.
References
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, et al. (1994) Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 3: 183-189.
- Dunlop GM (1989) A study of the relative frequency and importance of gastrointestinal symptoms, and weakness in patients with far advanced cancer: student paper. Palliat Med 4: 37-43.
- Whelan TJ, Mohide EA, Willan AR, Arnold A, Tew M, et al. (1997) The supportive care needs of newly diagnosed cancer patients attending a regional cancer center. Cancer 80: 1518-1524.
- Curtis EB, Krech R, Walsh TD (1991) Common symptoms in patients with advanced cancer. J Palliat Care 7: 25-29.
- Mock V, Atkinson A, Barsevick AM, Berger AM, Cimprich B, et al. (2007) Cancer-related fatigue. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 5: 1054-1078.
- Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, et al. (1994) Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum 21: 23-36.
- Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, et al. (2007) Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 105: 209-219.
- Curt GA (2000) Impact of fatigue on quality of life in oncology patients. Semin Hematol 37: 14-17.
- Spelten ER, Verbeek JH, Uitterhoeve AL, Ansink AC, van der Lelie J, et al. (2003) Cancer, fatigue and the return of patients to work-a prospective cohort study. Eur J Cancer 39: 1562-1567.
- Jereczek-Fossa BA, Marsiglia HR, Orecchia R (2002) Radiotherapy-related fatigue. Crit Rev Oncol Hematol 41: 317-325.
- Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, et al. (2004) Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer 40: 673-680.
- Bower JE (2005) Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol 23: 8280-8282.
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, et al. (2000) Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18: 743-753.
- Servaes P, Verhagen C, Bleijenberg G (2002) Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer 38: 27-43.
- Cheville AL (2009) Cancer-related fatigue. Phys Med Rehabil Clin N Am 20: 405-416.
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, et al. (2011) Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 29: 3517-3522.
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR (2006) Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12: 2759-2766.
- Cleare AJ, Messa C, Rabiner EA, Grasby PM (2005) Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol Psychiatry 57: 239-246.
- Payne JK (2006) The trajectory of biomarkers in symptom management for older adults with cancer. Semin Oncol Nurs 22: 31-35.
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, et al. (2009) Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology 34: 1476-1485.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR (2008) Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 26: 971-982.
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, et al. (2007) Mechanisms of cancer-related fatigue. Oncologist 12 Suppl 1: 22-34.
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, et al. (2006) Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 106: 751-758.
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, et al. (2009) Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res 15: 5534-5540.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC (2011) Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage 41: 761-767.
- Cramp F, Daniel J (2008) Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev: CD006145.
- Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, et al. (2011) Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20: 123-133.
- Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, et al. (2004) A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 100: 1302-1310.
- Windsor PM, Nicol KF, Potter J (2004) A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer 101: 550-557.
- de Oliveira Campos MP, Riechelmann R, Martins LC, Hassan BJ, Casa FB, et al. (2011) Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J Altern Complement Med 17: 505-512.
- Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G (2009) Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev: CD006953.
- Armes J, Chalder T, Addington-Hall J, Richardson A, Hotopf M (2007) A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer-related fatigue. Cancer 110: 1385-1395.
- Gielissen MF, Verhagen CA, Bleijenberg G (2007) Cognitive behaviour therapy for fatigued cancer survivors: long-term follow-up. Br J Cancer 97: 612-618.
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G (2006) Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol 24: 4882-4887.
- Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, et al. (2009) Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage 38: 650-662.
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, et al. (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85: 1186-1196.
- Wang XS, Janjan NA, Guo H, Johnson BA, Engstrom MC, et al. (2001) Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer 92: 1725-1732.
- Zick SM, Alrawi S, Merel G, Burris B, Sen A, et al. (2011) Relaxation acupressure reduces persistent cancer-related fatigue. Evid Based Complement Alternat Med 2011.
- Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, et al. (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89: 1634-1646.
- Wang XS, Laudico AV, Guo H, Mendoza TR, Matsuda ML, et al. (2006) Filipino version of the M. D. Anderson Symptom Inventory: validation and multisymptom measurement in cancer patients. J Pain Symptom Manage 31: 542-552.
- Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, et al. (2005) Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer 104: 1772-1778.
- Guren MG, Dueland S, Skovlund E, Fossa SD, Poulsen JP, et al. (2003) Quality of life during radiotherapy for rectal cancer. Eur J Cancer 39: 587-594.
- Janaki MG, Kadam AR, Mukesh S, Nirmala S, Ponni A, et al. (2010) Magnitude of fatigue in cancer patients receiving radiotherapy and its short term effect on quality of life. J Cancer Res Ther 6: 22-26.
- Schwartz AL, Nail LM, Chen S, Meek P, Barsevick AM, et al. (2000) Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest 18: 11-19.
- Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, et al. (2010) Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs 33: 201-212.
- Geinitz H, Thamm R, Scholz C, Heinrich C, Prause N, et al. (2010) Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlenther Onkol 186: 46-52.
- Sood A, Moynihan TJ (2005) Cancer-related fatigue: an update. Curr Oncol Rep 7: 277-282.
- Breetvelt IS, Van Dam FS (1991) Underreporting by cancer patients: the case of response-shift. Soc Sci Med 32: 981-987.
- de Jong N, Courtens AM, Abu-Saad HH, Schouten HC (2002) Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs 25: 283-297.
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, et al. (2004) Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage 28: 373-380.
- Schwartz CE, Sprangers MA (1999) Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med 48: 1531-1548.
- Schwartz AL (1998) Patterns of exercise and fatigue in physically active cancer survivors. Oncol Nurs Forum 25: 485-491.
- Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC (1998) Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum 25: 915-920.
- Nail LM (2001) Long-term persistence of symptoms. Semin Oncol Nurs 17: 249-254.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 7625
- [From(publication date):
November-2012 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 3058
- PDF downloads : 4567