Research Article Open Access
A Portable Microcontrolled Pumping Flow System Developed to In Loco Determination of Diuron at Nanomolar Levels Employing a Disposable Biosensor
Bruno C Janegitz*, Fernando Campanhã Vicentini, Vagner B dos Santos, Thiago B Guerreiro and Orlando Fatibello-Filho
Department of Chemistry, Center of Exact Sciences and Technology, Universidade Federal de São Carlos, São Carlos - SP, Brazil
- *Corresponding Author:
- Bruno C Janegitz
Department of Chemistry
Center of Exact Sciences and Technology
Universidade Federal de São Carlos
P. O. Box 676, 13560-970, São Carlos - SP, Brazil
Tel: +55-163-351-8098
Fax: +55-163-351-8350
E-mail: brunocj@ymail.com
Received date: February 24, 2015; Accepted date: May 19, 2015; Published date: May 26, 2015
Citation: Janegitz BC, Vicentini FC, Santos VB, Guerreiro TB, Fatibello-Filho O (2015) A Portable Microcontrolled Pumping Flow System Developed to In Loco Determination of Diuron at Nanomolar Levels Employing a Disposable Biosensor. J Anal Bioanal Tech 6: 247. doi: 10.4172/2155-9872.1000247
Copyright: © 2015 Janegitz BC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Microcontrolled systems coupled biosensors have attracted attention. Here, we describe an automated and portable microcontrolled pumping flow system (μC-PFS) developed to biosensing application. The flow system is fully automatic, portable, it works in battery module and it dispenses the use of microcomputer and the manual operations. This device presents mainly a microcontroller as central processing unit (CPU), an aquarium air pump and three-way solenoid valves to propulsion and the volume control of aliquots inserted in the flow system. Once available its performance, this device was used to pump samples and solution towards a compacted and miniaturized electrochemical flow cell (EFC) employing a multicommutated flow procedure. In the EFC, it was used a biosensor based on acetylcholinesterase immobilized on the gold nanoparticles modified-screen printed carbon electrode, which was used in order to perform the determination of diuron by enzymatic inhibition employing a chronoamperometry. Under the best experimental conditions, the calibration curve for diuron determination was linear in the concentration range from 80 to 1400 nmol/L with a detection limit of 50 nmol/L. Moreover, the proposed system was fully automated, showed precise, with low consumption of sample and waste generation with an analytical throughput of 13/h.
Keywords
Microcontrolled pumping; Biosensor; Disposable; Gold nanoparticles; Electrochemistry; Acetylcholinesterase
Introduction
Modern automatized systems have been proposed in order to develop procedures with high precision, accuracy and repeatability. In this context, improvements and upgrades in technologies for hardware, such as microcontroller and microprocessors, have revolutionized the analytical instrumentation [1,2]. Microcontroller is a modern device that presents an inner microprocessor and other peripherals as a clock, memory, lines of command for in/out data, registers, readers and analog-to-digital converter (A/D) [3]. This device is able to perform commands and instructions, which are driven by a code that can be loaded and storage into the memory of the microcontroller. In analytical chemistry, microcontrollers have been used for the construction of photometers, [4] spectrophotometers, [5] fluorimeters, [6] turbidimeters, [7,8] nephelometers, [8,9] potentiostats and luminometers [10]. In order to reach a full and more versatility automation, the microcontrolled instrumentation can be coupled to flow analyses procedures. Flow analyses with multicommutation procedures is very interesting because presents a good throughput with reducing of waste generation and consumption of reagent [4,9-11].
The use of nanoparticles in electroanalysis is in constant expansion [2,12,13]. In this context, several research groups have been proposed sensors and biosensors based on nanostructured materials [14-18], such as carbon nanotubes, graphene and metal nanoparticles [12]. In fact, particularity gold nanoparticles (AuNPs) exhibit electronic, optical and chemical properties very attractive for various technological applications such as nanoelectronics, sensors/biosensors, optics and catalysis [19].
Polyelectrolytes are long-chain macromolecules, produced by combining simple monomers, which have been applied in sensing and biosensing [20]. Poly(allylamine hydrochloride) (PAH) is a polyelectrolyte widely applied in sensing by producing stable composites films [21].
Inhibition based biosensor is widely used in the past years [22-24]. In this context, we can highlight the determination of pesticides [25], heavy metals [26], food preservatives nicotine [27], and other toxic compounds [28]. The inhibition can be characterized by the association and dissociation of inhibitors with the active site of the enzyme by reversible or irreversible processes. Enzymes such as acetylcholinesterase (AChE) have been performed in this biosensingtype. AChE has been used in biosensing to detect some kinds of pesticides, such as organochlorine and organophosphorus [29-34]. AChE is a serine esterase enzyme, which converts the acetylcholine neurotransmitter to choline and acetate after transmission of a nerve impulse [35].
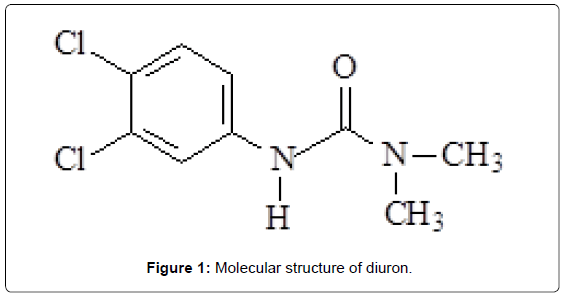
Diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] (Figure 1) is a organochlorine pesticide which is utilized in plantation of different products, e.g., coffee, sugar cane and cotton [36]. In humans, the exposition of this herbicide can produce methemoglobin in the blood and can cause problems in liver and spleen [37,38]. Some works have been related the determination of diuron using electroanalytical methods. Wong et al. [38] have proposed a biomimetic sensor based on a carbon paste electrode modified with the nickel (II) 1,4,8,11,15,18,22,25-octabutoxy-29H, 31H-phthalocyanine complex to detect diuron. They have obtained a linear range between 9.9 × 10-6 and 1.5 × 10-4 mol/L, and a detection limit of 6.1 × 10-6. A composite modified electrode with the thylakoid membrane isolated from spinach leaves was applied for amperometric detection of diuron, atrazine and propanil based on the photosynthesis-inhibiting [39]. The electrode presented detection limits for diuron, atrazine and propanil of 3.6 × 10-9, 6.1 × 10-9 and 8.7 × 10-9 mol/L, respectively. In another interesting work, Anh et al. reported a conductometric tyrosinase biosensor for the determination of diuron, atrazine, desisopropylatrazine and desethylatrazine [40].
Here, we describe a full automated and microcontroled pumping system with solenoid valves and aquarium pump as flow unit with a disposable screen printed carbon electrode (SPCE) modified with AuNPs and AChE coupled to portable potentiostat for amperometric determination of diuron pesticide in waters samples.
Experimental
Chemicals and reagents
Hydrogen tetrachloroaurate (III), 25% v/v glutaraldehyde, cystamine sulfate hydrate, acetylthiocholine iodide (ASCh), acetylcholinesterase ≥ 1,000 units/mg (from Electrophorus electricus) and diuron were purchased from Aldrich. PAH was purchased from Sigma. 0.01 mol/L stock solutions of these pesticides were prepared by dissolving appropriate amount in a 0.1 mol/L phosphate buffer solution (pH 7.0). Phosphate buffer solution was prepared using Na2HPO4 and NaH2PO4. All other chemicals were of analytical grade. All the solutions were prepared with Millipore Milli-Q nanopure water (resistivity>18 MΩ cm). The 0.1 mol/L phosphate buffer solutions were always employed as supporting electrolyte for biosensor measuraments.
Apparatus
A microcontrolled motherboard employing an embedded PIC18F4550 microcontroller (Dallas, USA) coupled to an aquarium air pump, 3 three-way solenoid valves were used to control the developed flow system. A peristaltic pump Ismate® (Glattbrugg, Switzerland) standard-speed, 8 channels, 115/230 VAC, EW-78001-12 was employed as reference pump. The voltammetric measurements were performed with SPCEs (4 mm of diameter) purchased from DropSens (Oviedo, Spain) were used, which include a silver pseudo-reference electrode and a carbon counter electrode. Electrochemical measurements were carried out by a DropSens potentiostat controlled by DropView 1.3 software. Cyclic voltammetry and amperometry measurements were performed in an electrochemical cell flow (Figure S1) developed for this purpose, with the void volume of 50 μl. All experiments were carried out at room temperature. A FEG-SEM (Supra 35-VP, Carl Zeiss, Germany) equipment with electron beam energy of 20 keV was employed to characterization of the biosensors developed.
Microcontrolled pumping flow system (μC-PFS)
A PIC18F4550 microcontroller from Microchip Technology® (Dallas, USA) was used as control and processing unit in the microcontrolled pumping flow system (μC-PFS). This device presents an analog-to-digital (A/D) multichannel converter with 10-bit resolution, 2.0 Kb of RAM; 256 bytes of EEPROM memory, 32.0 Kb of flash program memory; and an interface USB 2.0. The microcontroller sends pulses (5 V, 25 mA) to the ULN2003 (STMicroelectronics®, Italy) driver current in order to control the switching on/off the solenoid valves, aquarium air pump, and activation of the cooler. Moreover, the signal from the commands of the control buttons are received by the analog ports of the PIC and these data are used to control the switching time on the each solenoid valve set by the user. Recording of experimental data, and visualization of the time of switching on the valves on the LCD screen are others functions developed by the microcontroller. All the functions of the device are previously developed in C programming language [4,5,7-9].
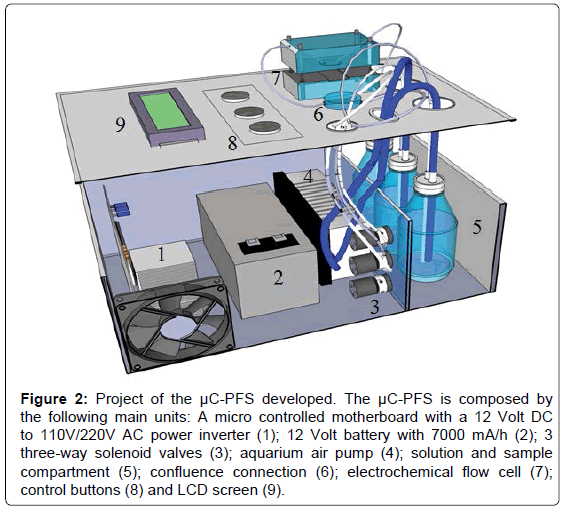
A project of the μC-PFS developed is shown in Figure 2 and a photography of μC-PFS with the disposable biosensor coupled to a potentiostat and a notebook (full portable analytical system) can be observed in the Figure S2 placed in the SI.
Figure 2: Project of the μC-PFS developed. The μC-PFS is composed by the following main units: A micro controlled motherboard with a 12 Volt DC to 110V/220V AC power inverter (1); 12 Volt battery with 7000 mA/h (2); 3 three-way solenoid valves (3); aquarium air pump (4); solution and sample compartment (5); confluence connection (6); electrochemical flow cell (7); control buttons (8) and LCD screen (9).
Software developed in C programming language
The software developed in C language carried out all functions of the device. Initially, the software performs a self-check of the digital and analog ports of the PIC, and after, it automatically initializes the control functions of the device. The functions control are chosen and selected using three keys on panel of the equipment. A message is then displayed on the LCD screen as a main menu containing information on the switching time of the reagent, sample/standard solution, and carrier solution (electrolyte) valve that should be executed by the equipment. The switching time in real time is displayed in the LCD screen of the μC-PFS.
Manifold and operation procedure of the μC-PFS
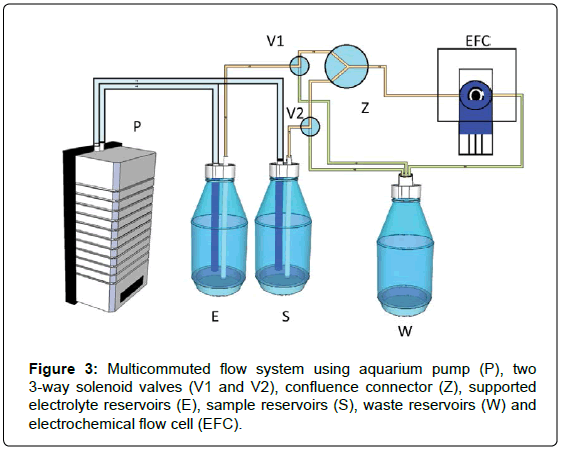
Firstly, the carrier solution (supporting electrolyte) is pumping from the proper reservoirs (E) by the aquarium air pump (P) with fixed flow and led to the 3-way solenoid valve (V1) which once switched on it changes the direction of flow in towards the electrochemical flow cell (EFC). Then, the base signal electrochemical is registered by the potentiostat and processed by the notebook plotting the data LCD screen. After the analyte (in supporting electrolyte medium) (V2) and the supporting electrolyte (V1) are pumping together until the confluence at Z point. The excess of sample (S), supported electrolyte and the waste are pumping toward the waste reservoir (W). The aquarium air pump presents three fixed flow rates, fast (76 μl/s), medium (47 μl/s), and low (26 μl/s) which are previously set by the user and controlled by the microcontroller.
The configuration of the flow system based on multicommutation with electrochemical detection is described as shown in the Figure 3. All switching time were determined previously by the user. All reservoirs used were of 500 ml. The device was used to pump solution towards a compacted and miniaturized electrochemical flow cell with a minibiosensor inserted in order to perform the determination of diuron employing voltammetric and chronoamperometric techniques.
Synthesis of AuNPs
The AuNPs colloidal solution was prepared by citrate reduction of HAuCl4 in aqueous solution. In a flask containing 150 ml of an aqueous solution of 5.0 mmol/L HAuCl4 at 90°C were added 7.5 ml of a solution of 0.3 mol/L sodium citrate. This mixture was kept stirring at 90°C until the appearance of red colour. Then, the reaction was rapidly cooled to room temperature with an ice bath.
Gold nanoparticles film
AuNPs film was prepared by dispersing 1.0 mg PAH in 1.0 ml of AuNPs colloidal solution. The dispersion was subjected to ultrasonication for 30 min. For comparison, a film of PAH without AuNPs was prepared by dispersing 1.0 mg of PAH in 1.0 ml of water. This dispersion was also subjected to ultrasonication for 30 min. Then, 8 μl of this AuNPs-PAH solution was cast on the working electrode of SPCE and waited for the solvent allowed evaporating at room temperature for 2 h. The electrode was designed AuNPs-PAH/SPCE.
Preparation of AChE-AuNPs/SPCE biosensor
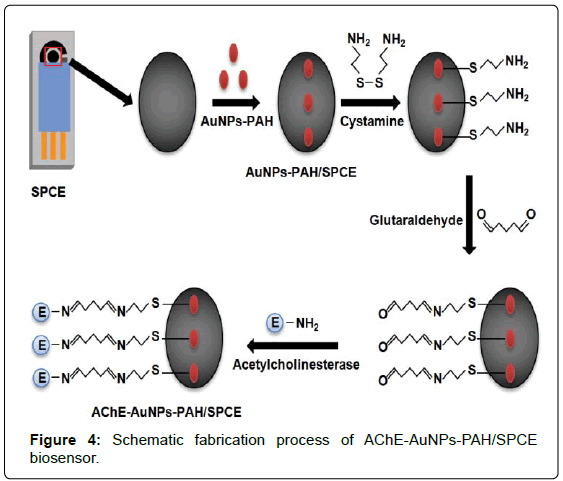
The AuNPs-PAH/SPCE were modified by casting 8 μl of 10 mmol/L cystamine solution on the electrode surface and the solvent was evaporated at room temperature for 1 h. The electrodes were rinsed with 0.1 mol/L phosphate buffer solution (pH 7.0). Then, 8 μl of 2.5% (v/v) glutaraldehyde solution was cast on the surface of the AuNPs- PAH/SPCE and the solvent was evaporated at room temperature for 1 h. The electrode was washed again with 0.1 mol/L phosphate buffer solution (pH 7.0). Finally, 8 μl of a solution containing 4 ml phosphate buffer solution (0.1 mol/L, pH 7.0) of 100 units of acetylcholinesterase was cast on the de surface of the AuNPs-PAH/SPCE and the evaporated at room temperature for 2 h. Thereafter, the electrode was thoroughly washed with phosphate buffer solution. The schematic fabrication process of biosensor was illustrated in Figure 4. When it was not in use, the AChE-AuNPs-PAH/SPCE was stored in a 0.1 mol/L phosphate buffer solution (pH 7.0) at 4°C.
Measurement procedure
For chronoamperometric measurements of diuron pesticide, AChE-AuNPs-PAH/SPCE biosensors were incubed in different concentrations of standard pesticide for 8 min, 0.1 mol/L phosphate buffer solution containing 1.0 mmol/L ASCh to study the electrochemical response. The inhibition percentage of pesticide was calculated as follows:
Inhibition %=(Is-Io) × 100%
where Is is the steady-state current of ASCh at the AChE-AuNPs- PAH/SPCE biosensor, and Io is the steady-state current of ASCh at the AChE-AuNPs-PAH/SPCE biosensor with pesticide inhibition.
It was used an electrode for each concentration of the pesticide. For the electrode substitution, the stopped flow procedure was applied for changing the biosensor for a new biosensor replaced. Furthermore, the baseline was corrected with the aid of software Origin® after receiving all amperometric signals.
Samples preparation
The samples of natural water were collected from Monjolinho lake dam at São Carlos, SP, Brazil. The recovery study using the proposed biosensor was performed by adding two different concentrations of diuron (50 and 1000 nmol/L) (using a solution 10 mmol/L diuron stock solution, prepared by dissolving in ethanol) to each sample. All samples were used without any pre-treatment and were freshly prepared for measurements. All waste generated were properly disposed in appropriate flasks and sent to Waste Management Unit at UFSCar for proper treatment.
Results and Discussion
Performance of the microcontrolled pumping flow system- μC-PFS
To the microcontroller performs the tasks efficiently, a crystal oscillator (clock) of 8 MHz was employed to synchronizing PIC functions with accuracy time. Once synchronized, the PIC, through the integrated circuit, (ULN2008) acts as a booster current switching on of each solenoid valve with 90 mA and 9.0 V. In order to use the microcontrolled pump in battery module, a rechargeable 12 V battery able to generate 7 A/h was employed. This battery was adapted on the electronic circuit supplying an electric autonomy to a lapse of 15 h which it is extremely useful to in situ analysis. However, the 110 or 220 V (conventional electric network, 60 Hz) also could be used as power supply to the device.
As the aquarium air pump [41] works in alternated current (AC), 60 Hz, an electronic circuit with a 12 Volt DC (direct current) to 110 V AC power inverter was required. Thus, an electronic circuit based on small transformer (input: 110-220 V AC, output, 12 V DC, 1 A) and an integrated circuit (CI) 555 set at a stable mode to produce an alternating signal of 110 V at 60 Hz of frequency was employed, with its activation was controlled by the PIC.
Plastic flasks with 500 ml of capacity were used as sample or solution reservoir, one for each channel (two channels) of the aquarium air pump. In upper side of the reservoirs (in cap), two holes were made to allow the introduction of two tubes polyethylene into the reservoir.
To prevent and avoid leakages and loss of pressure in the reservoirs, epoxy resin was used to seal the cap. Two different tubes were employed; a tube thicker (3.0 mm i.d.) was connected to the air pump to keep the pressurization, while the most thin (0.8 mm i.d.) was connect in the EFC.
The use of a microcontroller as CPU allows drastic reduction of the number of electronic components which can reduce the level of instrumental noise and cost of the developed equipment.
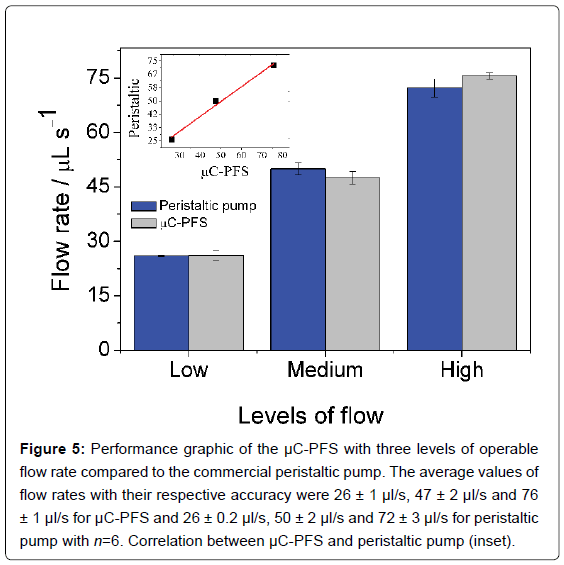
The aquarium air pump presents only two air outlets. These outlets were employed to pump the sample/standard solutions and supported electrolyte. According to previously discussed, the aquarium air pump operates with three fixed flow rates, fast (76 μl/s), medium (47 μl/s), and low (26 μl/s). To evaluate the performance of the portable μC-PFS developed, an Ismatec® peristaltic pump was employed as reference pump. The main results are presented in Figure 5.
Figure 5: Performance graphic of the μC-PFS with three levels of operable flow rate compared to the commercial peristaltic pump. The average values of flow rates with their respective accuracy were 26 ± 1 μl/s, 47 ± 2 μl/s and 76 ± 1 μl/s for μC-PFS and 26 ± 0.2 μl/s, 50 ± 2 μl/s and 72 ± 3 μl/s for peristaltic pump with n=6. Correlation between μC-PFS and peristaltic pump (inset).
The flow system based on multicommutation was simultaneously executed with the pump, and its performance was not impaired. In fact, there is a good accuracy with a minimum of pulsation of the solutions for each one of three levels of flow rate provided by μC-PFS as supported by the data showed. Besides, the performance of the μC-PFS was compared with the commercial peristaltic pump employing a similar flow rate and a good linear correlation (r=0.991) for the three levels of flow rate. In addition, a good performance (RSD of 1.06% for flow rate 76 μl) also was obtained for these flow rate described. Moreover, the μC-PFS presents a current consumption of 0.46 A/h by using a 7 A/h battery full changed. Thus, the μC-PFS has an electric autonomy to a lapse of 15 hours uninterrupted. Besides, for all essays, the level of the solution reservoirs, mainly the supported electrolyte should be always at least 10% (50 ml) of the maximum capacity (500 ml), since the internal pressure is reduced for this level of reservoir. For 26 μl/s, after 4.5 h uninterrupted, the flow is stopped and the reservoir should be filled again. In addition, higher volumes of plastic or glass reservoirs (e.g., 1.0 L) can be used, mainly for longer uninterrupted analytical procedures. However, for the present application in which a stopped flow manifold was employed, 500 ml reservoir was sufficient. After properly calibrated, the μC-PFS was applied to flow determination at electrochemical detection.
Characteristics of the AuNPs-PAH/SPCE
The electroactive area of SPCE and AuNPs-PAH/SPCE was estimated in 0.1 mol/L KCl in the presence of 1.0 mmol/L [Fe(CN)6]4- solution according to the Randles-Sevcik equation [42] (Equation 1).
Ip=2.69 × 105A D1/2 n3/2 v1/2 C (1)
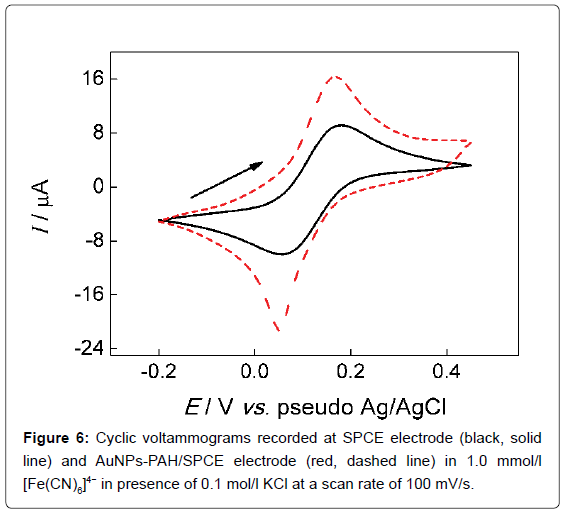
where Ip is the cathodic peak current (A), A is the electroactive area (cm5), D is the diffusion coefficient of [Fe(CN)6]4- in solution (6.2 × 10-6 cm2/s), n is the number of electrons transferred in the redox reaction, v is the potential scan rate (V/s), and C is the [Fe(CN)6]4- concentration in bulk solution (mol cm-3). The electroactive area of the SPCE and AuNPs-PAH/SPCE were calculated to be 0.044 cm2 and 0.095 cm2, respectively. As predicted observed in the literature, [43] the gold nanoparticles increased the area of the active electrode surface by a factor of 2.1 in comparison with the printed carbon electrode. In Figure 6 are shown the cyclic voltammograms at scan rate 100 mV/s, 0.1 mol/L KCl in the presence of 1.0 mmol/L [Fe(CN)6]4--.
The AuNPs colloidal solution was prepared by citrate reduction of HAuCl4 in aqueous solution, [44] as described in the section 4.8. The characterization of these metal nanoparticles was performed using the technique of SEM. The average particles diameter observed was 52 ± 5 nm as shown in the FEG-SEM images of AuNPs-PAH/SPCE at different magnitudes with the gold nanoparticles homogeneously distributed (Figure S3).
Electrochemical behaviour by cyclic voltammetry
When a biosensor contains the AChE immobilized, occurs the inhibition of production of the SCh [29] Thus, there is a relationship of the inhibition in presence of pesticides and the electrochemical signal decreases when compared in the absence and presence of the analyte of interest [29,45]. In the determination of organochlorine compounds, the inhibition of AChE activity is used and as a result there is a decrease of thiocholine (SCh) production. In the present study, the amperometric biosensor was based on the co-immobilization of AChE in AuNPs-PAH/SPCE, it means that the amount of SCh produced depends only on AChE activity, not ASCh concentration.
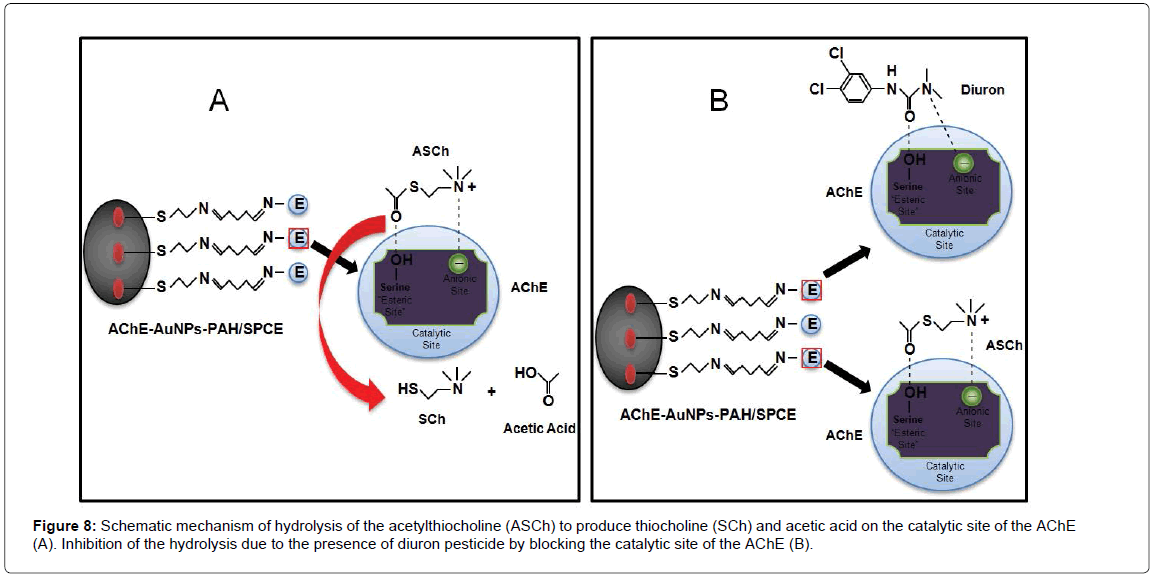
Figure 7 shows the typical cyclic voltammograms of AChE-AuNPs- PAH/SPCE at 50 mV/s in 0.1 mol/L phosphate buffer solution (pH 7.0) and 1.0 mmol/L ASCh, in absence (dashed line) and presence (solid line) of 1000 nmol/L diuron. It was observed a current peak at 0.75 V, which is attributed to the oxidation of SCh, hydrolysis product of ASCh. Obviously, in the presence of diuron pesticide in the AChEAuNPs- PAH/SPCE was observed a reduction of the signals due to the enzyme (AChE) inhibition, limiting the production of SCh. The mechanism of this inhibition is described in Figure 8.
As described, the AChE enzyme catalyzes the hydrolysis reaction of ASCh to form SCh and acetic acid [33]. This reaction can be inhibited, such as pesticides for the class of carbamates, organophosphates and organchlorines, which bind to serine active site of AChE. The binding is reversible with carbamates and is irreversible for organochlorines. Thus, the reactivation of the enzyme when bound AChE to organochlrine, although covalent, can be implemented employing some reagents e.g., pralidoxime and obidoxime [46]. Therefore, to perform the reactivation of the proposed biosensor would require a systematic study with these compounds to check the possibility of reuse of the biosensor in the determination of the diuron pesticide. However, this study was not necessary, since there was a replacement of the biosensor after use.
Study of inhibition time, pH and applied working potential
It was investigated the effect of the pH of 0.1 mol/L phosphate buffer solution between 4.0 and 8.0. A great analytical response at pH 7.0, which has been chosen for further experiments for detection of diuron.
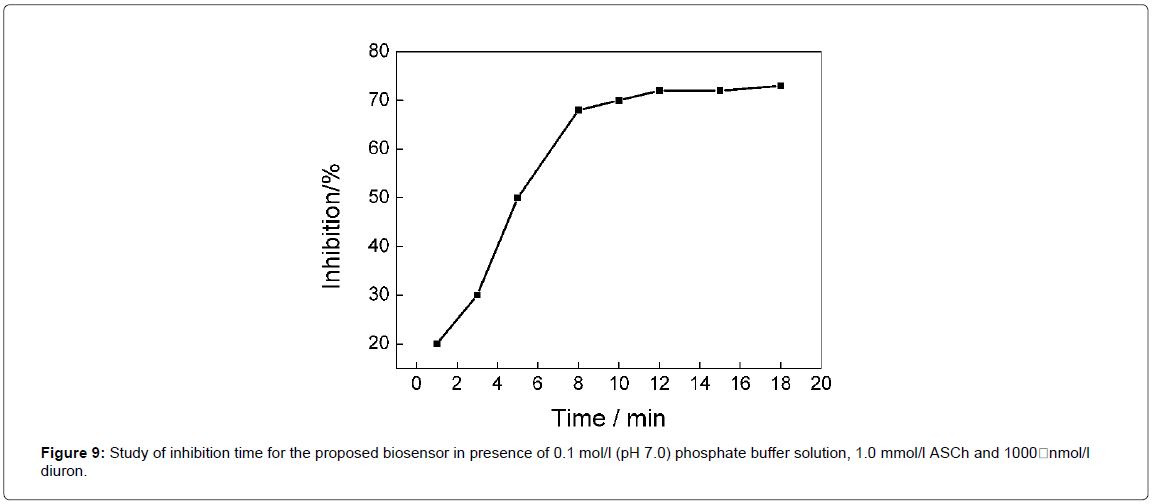
The influence of inhibition time on the current obtained was investigated in the range from 0 to 20 min in presence of 0.1 mol/L phosphate buffer, 1.0 mmol/L ASCh and 1000 nmol/L diuron, as observed in Figure 9. During this study, the μC-PFS was stopped flow after the arrival of the pesticide on the surface of the AChE-AuNPs- PAH/SPCE, and then, it has occurred the inhibition of formed product. The current decreased when there was an increase the time until 8 min, remaining constant for longer times. Thus, an inhibition time of 8 min was selected for further studies.
The effect of the applied working potential was also studied from 0.4 to 0.9 V vs. Ag pseudo-reference in presence of 0.1 mol/L phosphate buffer, 1000 nmol/L diuron and 1.0 mmol/L ASCh. It was observed a significant decrease of the analytical signal from 0.6 to 0.8, and, after, the current values practically remained constant. Therefore, the working potential of 0.8 V was selected for further studies.
Effect of the flow system parameter of the μC-PFS
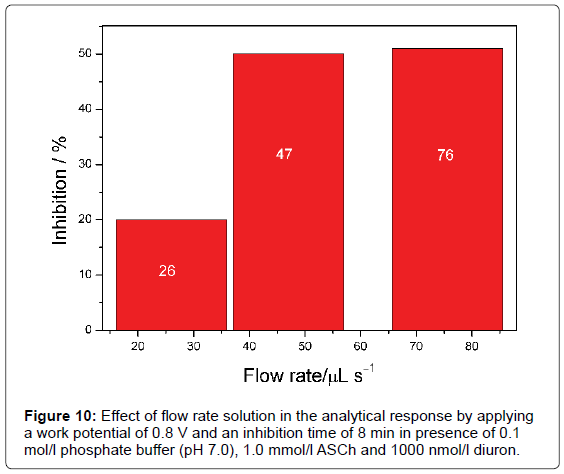
The effect of flow rate solution was investigated from 26 μl/s, 47 μl/s to 76 μl/s, applying a work potential of 0.8 V and an inhibition time of 8 min in presence of 0.1 mol/L phosphate buffer solution (pH 7.0), 1.0 mmol/L ASCh and 1000 nmol/L diuron. The current decreased with increasing flow rate up to 47 μl/s as observed in Figure 10. For higher flow rate (76 μl/s), the analytic signal has remained constant due to dispersion of the sample and a lower residence time of the electro active substance (thiocholine produced in the enzymatic reaction) in contact with the electrode surface. Therefore, a carrier solution flow rate of 47 μl/s was selected, keeping a good compromise between the magnitude of analytical signal and stability in the electrode response.
Analytical curve employing the μC-PFS with biosensor
Under the optimal experimental conditions an analytical curve was constructed. Thus, the inhibition process in the presence of different diuron concentrations was proportional in the current range from 80 to 1400 nmol/L (Figure S4) following the equation ΔIp (μA)=0.98+1.23 × 107 C (mol/L) with a correlation coefficient of 0.994. The detection limit was 50 nmol/L (calculated using the relationship 3 × Sd/m, where Sd is the standard deviation of various blank measures and m is the slope of the analytical curve). The electrode-to-electrode reproducibility response of five AChE-AuNPs-PAH/SPCE for a 100 nmol/L diuron solution was examined, which presented a standard deviation (RSD) of 5.4 % for five successive assays.
Interference studies for diuron determination
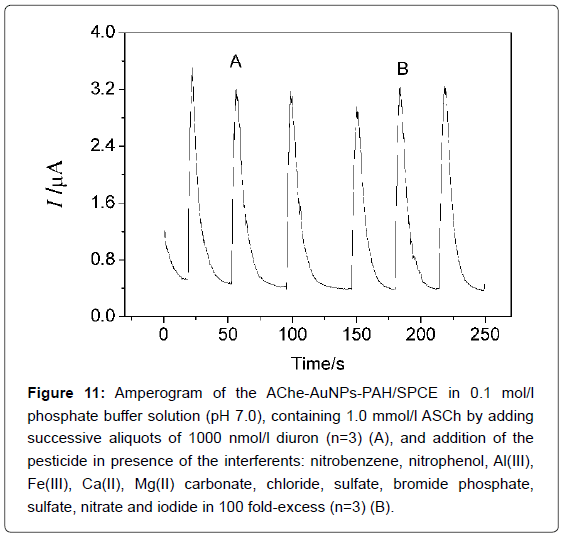
To evaluate the selectivity of the biosensor, the influence of some substances commonly found in water samples were evaluated in 0.1 mol/L phosphate buffer solution (pH 7.0), containing 1.0 mmol/L ASCh, in the presence of 1000 nmol/L diuron. The interference of compounds organic compounds such as nitrobenzene, nitrophenol and other inorganic ions such as, Al(III), Fe(III), Ca(II), Mg(II) carbonate, chloride, sulfate, bromide phosphate, sulfate, nitrate and iodide in excess of 100 times, caused interference less than 5% in the determination of diuron in water samples using the AChE-AuNPs- PAH/SPCE biosensor as observed in Figure 11.
Figure 11: Amperogram of the AChe-AuNPs-PAH/SPCE in 0.1 mol/l phosphate buffer solution (pH 7.0), containing 1.0 mmol/l ASCh by adding successive aliquots of 1000 nmol/l diuron (n=3) (A), and addition of the pesticide in presence of the interferents: nitrobenzene, nitrophenol, Al(III), Fe(III), Ca(II), Mg(II) carbonate, chloride, sulfate, bromide phosphate, sulfate, nitrate and iodide in 100 fold-excess (n=3) (B).
Determination of diuron in natural water samples
The water samples were collected from Monjolinho River (São Carlos, SP, Brazil) at three different points and, in each sample an appropriate amount of diuron was added. The recovery obtained of the samples A1, A2 and A3 varied between 94.0 and 107%, indicating the absence of the matrix interference effects in the samples studied using the proposed flow procedure. The results are shown in Table S1.
Conclusion
The μC-PFS portable presented as a good alternative and analytical tools for multicommuted flow system and flow analysis in general. Thus, the device developed has a great potential to be use in situ or in loco determination, once it has a good energetic autonomy. Additionally, as compared to peristaltic pump, the μC-PFS based on aquarium air pump has some advantages, such as lower cost, negligible pulsation, lower tendency to form bubbles in electrochemical flow cell, higher life time, great robustness and portability. According to the results demonstrated, the AuNPs-PAH was stable and reproducible. AChE could be effectively immobilized on the AuNPs, resulting enzyme electrode by exhibiting fast response, high sensitivity and stability for the amperometric detection of diuron. For outlook, the use of AuNPs-PAH onto SPCE is an interesting alternative to immobilize other biomolecules in future, also innovations and upgraded in order to apply the μC-PFS as approach for flow-batch procedures, flow injection analysis and sequential injection analysis.
Acknowledgement
We gratefully acknowledge the Brazilian funding agencies FAPESP (Proc. 2008/09893-0), CNPq, and CAPES for financial support and scholarships.
References
- Lapresta-Fernández A, Capitán-Vallvey LF (2011) Multi-ion detection by one-shot optical sensors using a colour digital photographic camera. Analyst 136: 3917-3926.
- Wang N, Zhang N, Wang M (2006) Wireless sensors in agriculture and food industry-Recent development and future perspective. Comput Electron Agric 50: 1-14.
- http://ww1.microchip.com/downloads/en/devicedoc/39632c.pdf
- Gaião Eda N, dos Santos SR, dos Santos VB, do Nascimento EC, Lima RS, et al. (2008) An inexpensive, portable and microcontrolled near infrared LED-photometer for screening analysis of gasoline. Talanta 75: 792-796.
- Veras G, Silva EC, Silva Lyra W, Soares SF, Guerreiro TB, et al. (2009) A portable, inexpensive and microcontrolled spectrophotometer based on white LED as light source and CD media as diffraction grid. Talanta 77: 1155-1159.
- Lam H, Kostov Y, Rao G, Tolosa L (2009) A luminescence lifetime assisted ratiometric fluorimeter for biological applications. Rev Sci Instrum 80: 124302.
- dos Santos VB, Guerreiro TB, Faria RC, Fatibello-Filho O, Suarez WT (2012) Construction and application of a portable microcontrolled turbidimeter for the in situ determination of sulfate. Quim Nova 35: 802-807.
- dos Santos VB, Guerreiro TB, Suarez WT, Faria RC, Fatibello-Filho O (2011) Evaluation of turbidimetric and nephelometric techniques for analytical determination of n-acetylcysteine and thiamine in pharmaceutical formulations employing a lab-made portable microcontrolled turbidimeter and nephelometer. J Braz Chem Soc 22: 1968-1978.
- dos Santos VB, Guerreiro TB, Suarez WT, Faria RC, Fatibello-Filho O (2011) A low-cost portable microcontrolled nephelometer for potassium determination. J Braz Chem Soc 22: 726-U186.
- Martínez-Olmos A, Ballesta-Claver J, Palma AJ, Valencia-Mirón Mdel C, Capitán-Vallvey LF (2009) A portable luminometer with a disposable electrochemiluminescent biosensor for lactate determination. Sensors 9: 7694-7710.
- Melchert WR, Reis BF, Rocha FR (2012) Green chemistry and the evolution of flow analysis. A review. Anal Chim Acta 714: 8-19.
- Campbell FW, Compton RG (2010) The use of nanoparticles in electroanalysis: an updated review. Anal Bioanal Chem 396: 241-259.
- Ai X, Wang Y, Hou X, Yang L, Zheng C, et al. (2013) Advanced oxidation using Fe(3)O(4) magnetic nanoparticles and its application in mercury speciation analysis by high performance liquid chromatography-cold vapor generation atomic fluorescence spectrometry. Analyst 138: 3494-3501.
- Welch CM, Compton RG (2006) The use of nanoparticles in electroanalysis: a review. Anal Bioanal Chem 384: 601-619.
- Janegitz BC, Pauliukaite R, Ghica ME, Brett CMA, Fatibello-Filho O (2011) Direct electron transfer of glucose oxidase at glassy carbon electrode modified with functionalized carbon nanotubes within a dihexadecylphosphate film. Sensors and Actuators B: Chemical 158: 411-417.
- Janegitz BC, Marcolino-Junior LH, Campana-Filho SP, Faria RC, Fatibello-Filho O (2009) Anodic stripping voltammetric determination of copper(ii) using a functionalized carbon nanotubes paste electrode modified with crosslinked chitosan. Sensor Actuat B-Chem 142: 260-266.
- Janegitz BC, Figueiredo-Filho LCS, Marcolino-Junior LH, Souza SPN, Pereira-Filho ER, et al. (2011) Development of a carbon nanotubes paste electrode modified with crosslinked chitosan for cadmium(II) and mercury(II) determination. J Electroanal Chem 660: 209-216.
- Sartori ER, Vicentini FC, Fatibello-Filho O (2011) Indirect determination of sulfite using a polyphenol oxidase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and gold nanoparticles within a poly(allylamine hydrochloride) film. Talanta 87: 235-242.
- Abollino O, Giacomino A, Malandrino M, Piscionieri G, Mentasti E (2008) Determination of mercury by anodic stripping voltammetry with a gold nanoparticle-modified glassy carbon electrode. Electroanalysis 20: 75-83.
- Kong H, Luo P, Gao C, Yan D (2005) Polyelectrolyte-functionalized multiwalled carbon nanotubes: Preparation, characterization and layer-by-layer self-assembly. Polymer 46: 2472-2485.
- Takeda HH, Janegitz BC, Medeiros RA, Mattoso LHC, Fatibello-Filho O (2012) Differential pulse voltammetric determination of ciprofibrate in pharmaceutical formulations using a glassy carbon electrode modified with functionalized carbon nanotubes within a poly(allylamine hydrochloride) film. Sensors and Actuators B: Chemical 161: 755-760.
- Pundir CS, Chauhan N (2012) Acetylcholinesterase inhibition-based biosensors for pesticide determination: a review. Anal Biochem 429: 19-31.
- Bachan Upadhyay LS, Verma N (2012) Enzyme inhibition based biosensors: A review. Anal Lett 46: 225-241.
- Van Dyk JS, Pletschke B (2011) Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 82: 291-307.
- Bucur B, Fournier D, Danet A, Marty JL (2006) Biosensors based on highly sensitive acetylcholinesterases for enhanced carbamate insecticides detection. Anal Chim Acta 562: 115-121.
- Ghica ME, Brett CA (2008) Glucose oxidase inhibition in poly(neutral red) mediated enzyme biosensors for heavy metal determination. Microchim Acta 163: 185-193.
- Sánchez-Paniagua López M, Hervás Pérez JP, López-Cabarcos E, López-Ruiz B (2007) Amperometric biosensors based on choline oxidase entrapped in polyacrylamide microgels. Electroanalysis 19: 370-378.
- Dzyadevych SV, Arkhypova VN, Martelet C, Jaffrezic-Renault N, Chovelon JM, et al. (2004) Potentiometric biosensors based on isfets and immobilized cholinesterases. Electroanalysis 16: 1873-1882.
- Shulga O, Kirchhoff JR (2007) An acetylcholinesterase enzyme electrode stabilized by an electrodeposited gold nanoparticle layer. Electrochem Commun 9: 935-940.
- Arduini F, Amine A, Moscone D, Palleschi G (2010) Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection (review). Microchim Acta 170: 193-214.
- Periasamy AP, Umasankar Y, Chen SM (2009) Nanomaterials - acetylcholinesterase enzyme matrices for organophosphorus pesticides electrochemical sensors: a review. Sensors (Basel) 9: 4034-4055.
- Pohanka M, Jun D, Kalasz H, Kuca K (2008) Cholinesterase biosensor construction - a review. Protein Pept Lett 15: 795-798.
- Schulze H, Vorlová S, Villatte F, Bachmann TT, Schmid RD (2003) Design of acetylcholinesterases for biosensor applications. Biosens Bioelectron 18: 201-209.
- Li Y, Han G (2012) Ionic liquid-functionalized graphene for fabricating an amperometric acetylcholinesterase biosensor. Analyst 137: 3160-3165.
- Sousa SCA, Rebelo MJF (2008) Acetylcholinesterase – choline oxidase biosensor for pirimicarb determination. Portugaliae Electrochimica Acta 26: 65-75.
- Cullington JE, Walker A (1999) Rapid biodegradation of diuron and other phenylurea herbicides by a soil bacterium. Soil Biology and Biochemistry 31: 677-686.
- Giacomazzi S, Cochet N (2004) Environmental impact of diuron transformation: a review. Chemosphere 56: 1021-1032.
- Wong A, de Vasconcelos Lanza MR, Sotomayor MDPT (2013) Sensor for diuron quantitation based on the p450 biomimetic catalyst nickel(ii) ,4,8,1,15,18,22,25-octabutoxy-29h,31h-phthalocyanine. J Electroanal Chem 690: 83-88.
- Li JP, Peng TZ (2004) Amperometric determination of trace amount of photosynthesis-inhibiting herbicides using a composite modified electrode with bioactive membrane. Acta Chim Sin 62: 1419-1424.
- Anh TM, Dzyadevych SV, Van MC, Renault NJ, Duc CN, et al. (2004) Conductometric tyrosinase biosensor for the detection of diuron, atrazine and its main metabolites. Talanta 63: 365-370.
- Matos RC, Gutz IGR, Angnes L, Fontenele RS, Pedrotti JJ (2001) A versatile and pulsation free pneumatic impeller for flow analysis systems. Quim Nova 24: 795-798.
- Bard AJ, Faulkner LR (2001) Electrochemical methods: Fundamentals and applications (2nd edition), John Wiley & Sons, New York, USA.
- Li J, Xie HQ, Li Y (2012) Fabrication of gold nanoparticles/polypyrrole composite-modified electrode for sensitive hydroxylamine sensor design. J Solid State Electrochem 16: 795-802.
- Gross S (2008) Colloidal dispersion of gold nanoparticles. Materials Syntheses 2008: 155-161.
- Turdean G, Popescu I, Oniciu L (2002) Amperometric cholinesterase biosensors for the determination of organophosphorus pesticides. Can J Chem-Rev Can Chim 80: 315-331.
- Pohanka M, Jun D, Kuca K (2008) Amperometric biosensors for real time assays of organophosphates. Sensors 8: 5303-5312.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15256
- [From(publication date):
June-2015 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 10572
- PDF downloads : 4684