Research Article Open Access
A Placebo-Controlled, Randomized Study on the Impact of Dietary Salmon Protein Hydrolysate Supplementation on Body Mass Index in Overweight Human Subjects
| Bomi Framroze1,*, Sanjay Vekariya2 and Dhruv Swaroop3 | |
| 1R&D Department, Hofseth Biocare AS. Molovegen, Norway | |
| 2Specturm Clinical Research, 37 New Marine Lines, Mumbai 400020, India | |
| 3GenePrint Health LLP, 4 Henry Road, Mumbai 400001, India | |
| Corresponding Author : | Bomi Framroze R&D Department, Hofseth Biocare AS Molovegen 6, 6004, Alesund, Norway Tel: 47 70 10 26 30 E-mail: bf@hofsethbiocare.no |
| Received: December 21, 2015; Accepted: January 12, 2016; Published: January 15, 2016 | |
| Citation: Framroze B, Vekariya S, Swaroop D (2016) A Placebo-Controlled, Randomized Study on the Impact of Dietary Salmon Protein Hydrolysate Supplementation on Body Mass Index in Overweight Human Subjects. J Obes Weight Loss Ther 6:296. doi:10.4172/2165-7904.1000296 | |
| Copyright: © 2016 Framroze B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
In the article, we show that a daily dietary intake of 16 g of salmon protein hydrolysate powder for 42 days statistically significantly reduced Body Mass Index by 5.6% in overweight subjects, in comparison with a placebo-control of whey protein isolate. Four metabolism-relevant serum biomarkers - bile acid, interleukin-6, Preheparin lipoprotein lipase mass and adiponectin - were also positively impacted using one-tailed, paired student t-test analysis, leading to the proposed metabolism-elevating mode-of-action. Exercise levels were maintained for each subject as per their normal levels. Further research will seek to identify the active individual peptide components from the salmon protein hydrolysate with specific biological activity for obesity control. Salmon protein hydrolysate powder in supplemental doses may be a useful tool in the long-term management of obesity.
| Keywords |
| Salmon; Protein; Hydrolystate; Obesity; Bile acid; Adiponectin |
| Introduction |
| Obesity is a major global health problem with over 3 million adults dying each year from obesity related complications [1]. Overweight and obese individuals with abnormal or excessive fat accumulation are at increased risk of type-2 diabetes, [2] cardiovascular disease [3] and metabolic disorders, particularly cancer [4]. |
| Most efforts to overcome obesity have focused on developing anti-obesity drugs such as fenfluramine for reducing energy intake, or hunger suppressants such as dexfenfluramine and satiety signalers such as sibutramine [5]. However, the few drugs that have entered the market are not widely used and are associated with serious side effects including insomnia, dry mouth, constipation, nausea and headache [6]. By contrast, functional foods are generally considered safe for clinical use, [7] and are being increasingly studied under clinically validated conditions. High whey-protein powders combined with low carbohydrate diets have been shown to reduce Body Mass Index (BMI) and increase metabolic responses [8]. A high-protein diet with casein has similarly shown a fat reducing effect, but had no impact on metabolic markers such as adiponectin, leptin and insulin, [9] while soy proteins have been shown to assist with dyslipidaemia and atherosclerosis prevention [10]. Most research efforts on marine protein hydrolysate powders such as those from krill [11] and fish [12] have focused on the reduction of plasma triacylglycerol levels and other cardiovascular biomarker benefits [13]. Some newer research has begun to expand the role of fish protein hydrolysates in metabolism and digestive modulation by showing an increase in plasma bile acid production [14], reducing adiposity [15], antihypertensive effect [16], antioxidant effect [17] and immunological effects [18] in animal and human trials. Other researchers have also shown the potential use of various protein hydro lysates in impacting metabolism and weight reduction. Morifuji et al. [19] have described an increase in muscle glycogen levels with whey hydro lysates probably related to the faster digestibility of the protein. Van Baak et al. [9] have shown that whey protein hydro lysates have modest fat mass reducing effects; but no relevant biomarkers, such as adiponectin or leptin, showed a corresponding metabolism increase correlation. More recently, Marette et al. [20] have shown that genetically modified mice fed a high-fat diet that included salmon protein hydrolysate (SPH showed a reduction in metabolic syndrome (MetS) as measured by glucose-intolerance and inflammation reduction. Significantly, all these studies only showed fat mass losses when the whey or fish protein hydro lysates were used as a significant component of the dietary protein source (50 g-140 g per day) and in conjunction with a low carbohydrate diet. |
| Our research has instead focused on identifying bioactive peptides present within SPH powder that may activate different metabolic pathways to increase fat burn at non dietary supplement dosing (4 g-16 g per day) when included as part of a normal diet. Our own recently published results on the ability of enzymatically hydrolyzed SPH powder to increase haemoglobin concentration in situationally anaemic human subjects at only 14 g per day supplemental dosing have shown the presence of such bioactive peptides already [21]. Based on this and our earlier encouraging results from animal studies, which showed an increase in bile-acid production in rats fed 2 g of SPH per day, we carried out this clinical trial to evaluate the potential antiobesity, increased fat burning effect of enzymatically liberated SPH protein powder on overweight human subjects and its effect on the four metabolism biomarkers shown below. |
| Plasma fatty acid (FA) composition is modulated by dietary intake and its composition has been associated with various health outcomes [22]. Bile acids are essential for solubilization of dietary fats [23]. Bile acids are synthesized in the liver and secrete through the biliary tree into the gall bladder. From the gall bladder the bile acids are secreted into the small intestine for digestion and are eventually reabsorbed and returned to the liver. Bile acids have been reported to reduce plasma and liver triacylglycerol (TG) levels in animal and human trials [24]. A high plasma concentration of TG is a characteristic feature of reduced metabolic activity and increased risk of Cardio-Vascular Disease (CVD). The reduction of plasma TG coupled with lowered metabolic activity via increased plasma BA is a relevant clinical goal to treat mild obesity [25]. Furthermore, glucose metabolism also seems to be regulated by increased circulatory bile acid [26]. Thus an increase in plasma bile acid levels could be viewed as a positive biomarker for the treatment of obesity. |
| Human Interleukin 6 (IL-6) is a 184 amino acid polypeptide. It is produced by various cells, including T- and B-cells, monocytes, fibroblasts, keratinocytes and endothelial cells. It regulates the growth and differentiation of various cell types with major activities in the immune system, metabolism, and inflammation [27]. Most normal controls have low levels of IL-6 in their serum. Increases of IL-6 are detected 97 in severe inflammatory situations, such as septicemia, and in metabolic and autoimmune diseases, such as arthritis and obesity [28]. The reduction of circulating IL-6 could be viewed as a biomarker to detect an improvement in underlying metabolic disorder during treatment for obesity. |
| Preheparin Lipoprotein lipase (Pr-LPL Mass) is a lipolytic enzyme involved in catalyzing the hydrolysis of triglycerides in chylomicrons and very low-density lipoprotein (VLDL) particles [29]. It is regulated by insulin and its serum level reflects insulin sensitivity [30]. Pr-LPL mass has also been used to assess insulin sensitivity in the general population and in advanced Type II diabetic patients [31]. Studies have shown that pre-heparin serum LPL mass has significant relationships with serum lipids and lipoproteins, visceral fat area, insulin resistance, and the development of CVD [32], and that an increase in its serum levels could be reflective of a decrease in visceral fat levels upon intervening dietary treatment for obesity. |
| The metabolic syndrome, a cluster of abdominal obesity, dyslipidemia, hypertension and hyperglycemia, is a common basis for atherosclerotic vascular diseases in industrial countries exposed to over nutrition [33]. Adiponectin is an adipose-derived plasma protein with anti-atherogenic and insulin-sensitizing activities [34]. Hypoadiponectinemia is closely associated with the clinical phenotype of metabolic syndrome and observing an elevated plasma concentration of adiponectin [35] may be a useful biomarker for measuring the effectiveness of dietary treatment for obesity. |
| The objective of this study was to measure the change in fat burn through Body Mass Index (BMI) without any change in the level of exercise for each subject and observe any changes in the related metabolic biomarkers of bile acid, interleukin-6, preheparin lipoprotein lipase mass and adiponectin after 42 days of SPH powder supplementation in overweight people using a randomized, placebocontrolled protocol. |
| Materials and Methods |
| Study design and Subjects |
| The objective of this prospective, open, randomized study was to determine the dietary effects of salmon protein hydrolysate on decreasing BMI with commensurate modulation of circulatory levels of the metabolic biomarkers of bile acid, interleukin-6, preheparin lipoprotein lipase mass and adiponectin in overweight subjects. This study was conducted in accordance with the principles of the Declaration of Helsinki guidance for good clinical practice, Seoul 2008 and Good Clinical Practices guidelines for clinical research in India, ICMR. The final approved protocol and all the study related documents were reviewed and approved by the ClinXXL Independent Ethics Committee before the start of the study. |
| Salmon protein hydrolysate tablets were sourced from Pharmatech AS, Rolvsoy, Norway and Whey Protein Hydrolysate powder was sourced from Protein Fabrikken AS, Stokke, Norway. 48 human subjects between the ages of 18 and 65 with overweight BMI values between 25 and 30 were recruited for the study. 143 Subjects who were already on specific diet recommendations, on caloric reduction diets or other special diets were excluded from this study. Subjects who had uncontrolled diabetes, coronary artery disease, cardiovascular disease and coronary atherosclerosis or uncontrolled hypertension were also excluded. Subjects with clinical signs and symptoms of liver, kidney or thyroid disorder and tuberculosis were also excluded. Alcoholics, smokers, and pregnant and lactating women were also excluded from this study. |
| Experimental design and procedures |
| The total duration of this study was 42 days. A total of 48 subjects of which female (n=37) and male (n=11) were recruited in the study. The 48 subjects were split into two study groups. Group 1 (G1) consisted of 24 overweight subjects with BMI between 25 and 30 who received 16 x 1 gram tablets of SPH to be taken daily at breakfast while maintaining their routine diets. Group 2 (G2) consisted of 24 overweight subjects with BMI levels between 25 and 30 who received 18 g powder sachets of whey protein isolate (WPI) to be taken daily at breakfast while maintaining their routine diets (Table 1). |
| Dietary counselling was carried out to normalize the subject pool between the two groups so that there was no significant calorie and type of foods difference in diet between them. A detailed history of food consumption was taken based on a 24-hour dietary recall method. The complete dietary history included the consumption of vegetarian and non-vegetarian food and the frequency of consumption of fish or meat was obtained for each subject. Standardized containers were used to assess the amount of food consumed. The average daily intake of major food groups, namely cereals, pulses, meat, milk, vegetables, fruits and oil was assessed, and subjects who were consuming oily rich food regularly were advised to limit the same during the study. The subjects were instucted to follow similar diets during the course of the study and to note any significant deviations during the twice weekly telephone follow-up. |
| At Visit 1 (Clinic Visit 1/Day -2), the pre-enrolment clinical safety assessment was carried out, which included informed consent signing, medical history review, and systemic and physical examination for all screened subjects. The subjects were only enrolled in the study after confirmation of study inclusion and exclusion criteria. |
| During the baseline visit (Clinic Visit 2/Day 0), the subjects were given their salmon protein tablets or their whey protein powder sachets for the study and dietary counselling was repeated by trained dieticians. Telephonic follow up for routine diet monitoring and protein hydrolysate tablets/sachet intake compliance was performed twice weekly for the entire duration of the study. |
| On the final day of the study (Clinic Visit 3/187 Day 42), a systemic and physical examination was carried out for each subject and a 10 ml blood sample taken for analysis of the metabolic biomarkers, bile acid, interleukin-6, preheparin lipoprotein lipase mass and adiponectin. Bile acid was measured using the colorimetric method and kits supplied by Cell Bio labs Inc. USA. Serum Interleukin-6 levels were analyzed using the ELISA kit supplied by Life Technologies Inc. USA. Preheparin lipoprotein lipase mass was analyzed using the ELISA kit supplied by ALPCO Inc. and adiponectin was measured using the ELISA kit and method supplied by Cayman Chemicals Inc. USA. |
| Among the recruited subjects, 1 subject was prematurely discontinued from the study due to reasons not related to the protein powder. A total of 47 subjects completed the study as per the protocol. |
| Results |
| All raw data from this study was analyzed using “Sigma Plot 11.0” statistical software (Supplied by Cranes Software International Ltd. Bangalore). The mean and standard deviation was calculated using Microsoft Excel Sheets and all data summarized in tabular form. Data describing quantitative measures were expressed as mean, SD with range. Changes in variables were estimated by analysis of variance. All p values were reported based on two sided significance test and all statistical tests were interpreted at 95% level of significance. No treatment related clinical signs or symptoms were observed in any of the subjects at the end of the study period. 19 of the 23 subjects that completed the trial in G1 showed 209 a significant decrease in BMI levels such that the mean BMI level for G1 showed a statistically significant 5.9% decrease from baseline at Day 42. Only 6 out of the 24 subjects in G2 showed a very modest decrease in BMI levels such that the decrease in mean BMI level for G2 was not statistically significant, as shown below in Table 2. |
| The plasma bile acid levels were found to be significantly increased for the SPH treated group G1 versus the WPI treated group G2 as shown below in Table 3. At the end of the 42-day study, the SPH test group showed a statistically significant increase in plasma bile acid concentration (from a normal baseline of 5.7 uM/L to a 9.3 uM/L) as compared to the WPI control group, which did not show a statistically significant shift (from 6.6 uM/L to 6.9 uM/L). The mean serum IL-6 levels were found to be at the upper end of the normal range as compared to the literature (2.0 pg/ml +/- 1.5) for both groups of patients at the start of the study. At the end of the 42 day study, the SPH treated test group G1 showed a statistically significant decrease in IL-6 concentration (from 3.4 pg/ml to 2.9 pg/ml) as compared to the WPC control group G2, which did not show a statistically significant decrease as shown below in Table 4. |
| The serum Pr-LPL Mass levels were measured using 241 an ALPCO ELISA kit assay and were found to be statistically significantly increased for the SPH treated group G1 versus the WPI treated group G2 as shown below in Table 5. At the end of the 42-day study, the SPH test group showed a statistically significant (90% confidence level) increase in Pr-LPL Mass concentration (from a normal baseline of 62.2 ng/ml to 71.3 ng/ml) as compared to the WPI control group, which did not show a statistically significant shift (from 66.7 ng/ml to 68.4 ng/ml). A larger or longer study would be needed to repeat this statistical Pr-LPL Mass concentration increase and correlate this to insulin sensitivity changes. |
| The serum adiponectin levels were found to be significantly increased for the SPH treated group G1 versus the WPI treated group G2 as shown below in Table 6. At the end of the 42-day study, the SPH test group showed a statistically significant (90% confidence level) increase in adiponectin concentration (from a normal baseline of 6.2 ug/ml to 6.9 ug/ml) as compared to the WPI control group, which did not show a statistically significant change. |
| Discussion |
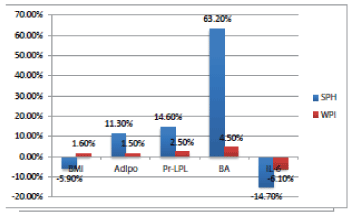
| The standard of care for overweight individuals is to suggest exercise and diet, which, if ineffective, quickly leads to clinical obesity, defined as a BMI greater than 30. At this point, the standard of care remains diet and exercise, but doctors are increasingly depending on either invasive surgery or drugs which have a myriad of side-effects [36] and often result in a relapse of weight-gain. Multiple studies have shown that both treatments may not result in long-term improved BMI and weight loss for a significant number of patients [37]. The use of functional foods to decrease BMI has seen increased research attention [38] and specific modes of action for these foods have focused on insulin regulation [39], gut micro biota rebalance [40] and modulating the metabolic syndrome [41]. One aspect of our results shows that after 8 weeks of daily, supplemental-dose administration of salmon protein hydrolysate powder in overweight subjects, it may be concluded that salmon protein hydrolysate significantly lowered BMI in overweight subjects. Our results further showed that metabolism related, circulatory biomarkers - bile acid, adiponectin, Pr-LPL Mass and Interleukin-6 - showed positive improvements, indicating that the SPH lowers BMI via interaction with the metabolic pathways. As can be seen in Figure 1, salmon protein hydrolysate, at a dose of 16 g per day, showed a statistically significant decrease in BMI and positive impacts on the key metabolic syndrome biomarkers. By contrast the whey protein isolate control had no lowering of BMI nor any significant impact on these biomarkers. This implies that the decrease in BMI may be related to a modulation of inflammation and metabolism pathways, possibly via the presence of bioactive peptides in the SPH. It is noteworthy to mention here that 83% of the subjects in the SPH treated group G1 showed a significant decrease in BMI, while only 25% showed even a modest decrease in the WPI treated G2 group. |
| In this initial study, we have not evaluated certain parameters that do limit the conclusions that can be drawn. We have not identified an optimum dose/period for effective treatment, or how long the effect of the SPH supplementation may last, and have not directly measured the insulin and leptin levels to better understand sugar metabolism and appetite signalling during SPH supplementation. We are planning on fractionating the 641 peptides present in the SPH powder by both sizeocclusion column as well as ion-exchange chromatography to further understand the potential role played by individual bioactive peptides. This remains an active area of research in our laboratory. |
| Our current results clearly show that dietary supplementation with 16 g of salmon protein hydrolysate per day decreases BMI in overweight individuals and positively impacts circulatory biomarkers associated with inflammation and metabolism, within only 8 weeks of treatment. |
References
- (2015)World Health Organization. Obesity and overweight. Fact sheet No 311.
- Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ (2015) Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials.J AcadNutr Diet 115: 1447-1463.
- Cote AT, Phillips AA, Harris K, Sandor G, Panagiotopoulos C, et al. (2015) Obesity and Arterial Stiffness in Children: Systematic Review and Meta-Analysis. Arteriosclerosis Thrombosis and Vascular Biology 35: 1038-1044
- Iyengar NM, Hudis CA, Dannenberg AJ (2015) Obesity and cancer: local and systemic mechanisms.Annu Rev Med 66: 297-309.
- Halford JC, Boyland EJ, Lawton CL, Blundell JE, Harrold JA (2011) Serotonergic anti-obesity agents: past experience and future prospects.Drugs 71: 2247-2255.
- Harrison-Woolrych M, Clark DW, Hill GR, Rees MI, Skinner JR (2006) QT interval prolongation associated with sibutramine treatment.Br J Clin Pharmacol 61: 464-469.
- Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF (2015) A natural solution for obesity: bioactives for the prevention and treatment of weight gain. A review.NutrNeurosci 18: 49-65.
- Layman DK (2004) Protein quantity and quality at levels above the RDA improves adult weight loss.J Am CollNutr 23: 631S-636S.
- Claessens M, van Baak MA, Monsheimer S, Saris WH (2009) The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors.Int J Obes (Lond) 33: 296-304.
- Sirtori CR, Galli C, Anderson JW, Arnoldi A (2009) Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins.Atherosclerosis 203: 8-17.
- Ramsvik MS, BjorndalB, Vik R, Bruheim I, Skorve J, et al. (2013) Krill protein hydrolysate reduces plasma triacylglycerol level with concurrent increase in plasma bile acid level and hepatic fatty acid catabolism in high-fat fed mice. Functional Foods in Health and Disease 3: 428-440
- Wergedahl H, Liaset B, Gudbrandsen O, Leid E, Espe M, et al. (2004) Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol and lowers acyl-CoA:cholesterolacryltransferase activity in liver of Zucker rats J Nutr 13: 1320-1327
- Hosomi R, Fukunaga K, Arai H, Kanda S, Nishiyama T, et al. (2011) Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption.J Food Sci 76: H116-121.
- Shahidi F, Ambigaipalan P (2015) Novel functional food ingredients from marine sources. Current Opinion in Food Science 2: 123-129
- Pilon G, Ruzzin J, Rious L, Lavigne C, White P, et al. (2011) Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status and insulin sensitivity in high-fat fed rats. Metabolism Clinical And Experimental 60: 1122-1130
- Lee S H, Qian Z, Kim S (2010) A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chemistry 118: 96-102
- Godinho I, Pires C, Pedro S (2015) Antioxidant Properties of Fish Protein Hydrolysates Prepared from Cod Protein Hydrolysate by Bacillus sp.ApplBiochemBiotechnol .
- Boutin Y, Paradis M, Couture P, Lamarche B (2012) Immunilogical effects of fish protein supplementation on healthy adults. Journal of Natural Products 5: 37-44
- Morifuji M, Sakai K, Sanbongi C, Sugiura K (2005) Dietary whey protein increases liver and skeletal muscle glycogen levels in exercise-trained rats.Br J Nutr 93: 439-445.
- Chevrier G, Mitchell P, Rioux L, Hasan H, Jin T, et al. (2015) Low molecular weight peptides from salmon protein prevent obesity-linked glucose intolerance, inflammation and dyslipidemia in LDLR/ApoB Mice. Journal of Nutrition 145: 1415-1419
- Framroze B, Vekariya S, Swaroop D (2015) A Placebo-Controlled Study of the Impact of Dietary Salmon Protein Hydrolysate Supplementation in Increasing Ferritin and Hemoglobin Levels in Iron-Deficient Anemic Subjects. J Nutr Food Sci 5: 379-382
- Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, et al. (2014) Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis.Ann Intern Med 160: 398-406.
- Li T, Chiang JY (2014) Bile acid signaling in metabolic disease and drug therapy.Pharmacol Rev 66: 948-983.
- Porez G, Prawitt J, Gross B, Staels B (2012) Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease.J Lipid Res 53: 1723-1737.
- Prinz P, Hofmann T, Ahnis A, Elbelt U, Goebel-Stengel M, et al. (2015) Plasma bile acids show a postive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Frontiers in Neuroscience 9: 1-10
- Staels B, Prawitt J (2013) Soaping up type 2 diabetes with bile acids?: the link between glucose and bile acid metabolism in humans tightens: quality matters!Diabetes 62: 3987-3989.
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M (2012) IL-6/IL-6 receptor system and its role in physiological and pathological conditions.Clin Sci (Lond) 122: 143-159.
- Luckik L, Lalic N, Rajkovic N, Jotic A, Lalic K, et al. (2014) Hypertension in obese type 2 diabetes pateints is associated wih increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health 11: 3586-3598
- Shiba C, Shiba T, Takahashi M, Hori Y, Maeno T (2014) Relationships among serum lipoprotein lipase mass, visceral fat and retinal nerve fibre layer thickness. GraefeasArch Clin ExpOpthalomol 253: 1883-1888
- Kobayashi J (2004) Pre-heparin lipoprotein lipase mass.J AtherosclerThromb 11: 1-5.
- Kobayashi J, Miyashita K, Nakajima K, Mabuchi H (2015) Hepatic Lipase: a Comprehensive View of its Role on Plasma Lipid and Lipoprotein Metabolism.J AtherosclerThromb 22: 1001-1011.
- Kobayashi J, Saito K, Fukamachi I, Taira K, Takahashi K, et al. (2001) Pre-heparin plasma lipoprotein lipase mass: correlation with intra-abdominal visceral fat accumulation.HormMetab Res 33: 412-416.
- Grundy SM (2012) Pre-diabetes, metabolic syndrome, and cardiovascular risk.J Am CollCardiol 59: 635-643.
- Haar L, Luther K, Rubinstein J, Ren X, Jones W (2015) Adiponectin modulates NF-kB mediated cardioprotective pathways after acute high-fat feeding. J Fed Am SocExpBiol 29: 942-946.
- Ohashi K, Ouchi N, Matsuzawa Y (2012) Anti-inflammatory and anti-atherogenic properties of adiponectin.Biochimie 94: 2137-2142.
- Kang JG, Park CY (2012) Anti-Obesity Drugs: A Review about Their Effects and Safety.Diabetes Metab J 36: 13-25.
- Livhits M, Mercado C, Yermilov I, Parikh J, Dutson E, et al. (2012) Preoprative predictors of weight loss following bariatric surgery: Systematic review. Clinical Research Obesity Surgery 22: 70-89
- Baboota R, Bishnoi M, Ambalam P, Kondepudi K, Sarma S, et al. (2013) Functional food ingredients for the management of obesity and associated co-morbidities A review. J Functional Foods 5: 997-1012
- KazeemM (2015) Anti-diabetic functional foods as sources of insulin secreting, insulin sensitizing and insulin mimetic agents. J Functional Foods 5: 122-138.
- Mozzi F, Ortiz M, Bleckwedel J, Vuyst L, Pescuma M (2013) Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res Intl 54: 1152-1161.
- Suhaila M (2014) Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovascular disease. Trends in Food SciTech 35: 114-128
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 11625
- [From(publication date):
February-2016 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 10697
- PDF downloads : 928
