A Pilot Study of Nemonoxacin in Patients with Diabetic Foot Infections
Received: 13-Nov-2018 / Accepted Date: 04-Mar-2019 / Published Date: 11-Mar-2019 DOI: 10.4172/2332-0877.1000397
Abstract
Background: Diabetic foot infections (DFIs) are usually caused by a mixture of bacterial pathogens, especially staphylococci (including methicillin-resistant strains). Nemonoxacin, a broad-spectrum non-fluorinated quinolone with activity against methicillin-resistant Staphylococcus aureus (MRSA), could potentially be an effective agent to treat DFIs. The efficacy, safety, pharmacokinetics, and pharmacodynamics of nemonoxacin were evaluated in patients with DFI. Methods: Patients with mild or moderate DFI were treated with nemonoxacin 750 mg orally once daily for 7-14 days in this open-label, single-arm, multi-center study. Clinical and microbiological responses were evaluated. Blood and tissue samples were collected for assessment of the ability of nemonoxacin to penetrate into infected soft tissue wounds. Results: Thirty-eight patients were enrolled, 25 successfully completed the study. The predominant wound isolate was Staphylococcus aureus (in 69.7%), four of which were MRSA. Clinical success rate in evaluable patients at the test-of-cure (TOC) visit was achieved in 95.7% of patients in the intent-to-treat (ITT) and 94.7% in the per-protocol (PP) populations. Microbiological success rate at TOC in ITT and PP populations were 82.6% and 89.5%, respectively. Wound healing response (a validated wound score) demonstrated that the severity of infection was substantially reduced after treatment. Treatment with nemonoxacin was well tolerated. Nemonoxacin was rapidly absorbed and distributed to soft tissue following oral administration, with Cmax,ss attained at ~2 hours after dosing. Drug concentrations in soft tissue were >2.5 times of that in plasma at most sampling points. The ratio of AUC0-24 (tissue/plasma) was 3.08, with fAUC/MICs in plasma ranging from 13.1 to 1747.9, while AUC/MICs in soft tissue were 48.0 to 3200.0. Conclusion: In this small sample of DFI patients, nemonoxacin demonstrated good clinical and microbiological success rates, was well tolerated, and penetrated extensively into infected tissues. These results suggest that oncedaily oral nemonoxacin may be suitable for treating patients with DFI. ClinicalTrials.gov identifier: NCT00685698.
Keywords: Nemonoxacin; Diabetic foot infections; DFI; Fluoroquinolone; Efficacy; Safety
Introduction
Foot infections in persons with diabetes are a major and increasing problem throughout the world, especially in developing countries in Africa, Asia, and South America [1]. There are currently an estimated 451 million people with diabetes worldwide, and this number is expected to rise to 693 million by 2045 [2]. Foot ulceration is one of the most common and morbid complications of diabetes, with an annual incidence of about 6.3%, and a lifetime incidence as high as 34% [3]. Over half of these foot ulcerations become infected, and 20% of patients with infected foot wounds require some type of lower extremity amputation [4-6].
Most diabetic foot infections (DFIs) are caused by a mixture of bacterial pathogens. While aerobic gram-positive cocci, such as staphylococci and streptococci, are predominant in most infections, gram-negative bacilli and obligate anaerobic pathogens are also frequently isolated [7-9]. Among the diabetic foot pathogens, Staphylococcus aureus is the most common, and many strains are resistant to commonly used antibiotics [8,10,11]. Methicillin-resistant Staphylococcus aureus (MRSA) strains are now a common cause of DFIs, with a prevalence of 15-30% [10,12,13]. These may be associated with a particularly high rate of surgical debridement and amputation [13]. Furthermore, a recent retrospective study showed that 55% of MRSA isolates from diabetic foot ulcers were resistant to 7 to 10 antibiotics, suggesting that currently used antibiotics may often be ineffective for treating DFIs associated with MRSA [14]. Thus, newer antibiotics active against these resistant organisms are needed.
Nemonoxacin (TG-873870) is a nonflourinated quinolone antibiotic with a remarkably board-spectrum of antimicrobial activity. In vitro activity studies have demonstrated that nemonoxacin, when compared with levofloxacin and moxifloxacin, has greater activity against grampositive cocci, and comparable activity against gram-negative bacilli [15]. Furthermore, unlike other currently available fluoroquinolone agents, nemonoxacin is highly active against most antibiotic-resistant strains, e.g. MRSA, penicillin-and quinolone-resistant Streptococcus pneumoniae (PRSP and QRSP) and vancomycin-resistant enterococci [16,17].
In clinical studies, nemonoxacin exhibited high clinical cure and microbiological success rates in patients with community-acquired pneumonia, showing high activity against the common causative pathogens and good tolerability [18-20]. Thus, nemonoxacin, with its board-spectrum of antimicrobial activity, particularly against grampositive cocci, may be an effective agent for treating DFIs. This pilot study was designed to investigate the efficacy and safety of once daily orally administered nemonoxacin for treating mild to moderate diabetic foot infection. In addition, the ability of nemonoxacin to penetrate effectively into infected wounds and the pharmacokinetic and pharmacodynamic (PK/PD) properties of nemonoxacin in these DFI patients were also evaluated.
Materials and Methods
Study design
This was an open-label, single-arm, multi-center study in patients with a mild or moderate (by the Infectious Diseases Society of America [IDSA] classification) DFI caused by at least one gram-positive pathogen. Nemonoxacin was dispensed in 7-day cycles, instructing the patient to take 750 mg orally once-daily for 7 ± 1 or 14 ± 1 day, according to the discretion of the investigator. The dose of nemonoxacin was selected based on AUC/MIC90, Cmax/MIC90, and safety data obtained from previous phase I study [21-23]. Continuous treatment was allowed for a maximum of 28 ± 1 day if, in the investigator’s judgment, the patient was likely to benefit from additional therapy. The primary objective of this study was to assess the clinical outcome of treatment with nemonoxacin in patients with DFI. The study was conducted in compliance with good clinical practice and the Declaration of Helsinki. Institutional review boards of each participating site approved the study protocol and all subjects provided written informed consent before being enrolled in the study.
Eligibility criteria
Diabetic patients (receiving any type of glycemic treatment regimen) were eligible for this study if they were at least 18 years of age, weighed ≥ 40 kg, had a hemoglobin A1c level of ≤ 12%, had any type of infection of the foot that met the IDSA guideline criteria for mild or moderate, and had a wound culture that grew at least one grampositive pathogen. The found wound had to allow for the investigator to obtain a suitable tissue specimen for Gram-stained smear (to confirm the presence of gram-positive bacteria) and bacterial culture.
Patients were excluded from the study if in the opinion of the investigator they had: life expectancy of less than 6 months; a comorbid condition that could compromise evaluation of the foot infection or participation in this study (e.g., severe hepatic disease, renal failure, active systemic malignancy); possible involvement of the underlying bone (osteomyelitis) or joint (septic arthritis); known or suspected critical ischemia of the affected limb; a clinically significant cardiac electrical conduction abnormality or a history of prolonged QTc interval; a hypersensitivity reaction to any quinolone antibiotic; current alcohol abuse or illicit drug use; or, a seizure disorder. Also excluded were subjects who were pregnant, lactating, or fertile and not using contraception; had a neutrophil count < 1000 cells/mm3; were significantly immunosuppressed or had undergone chemotherapy within the previous 6 months or would need such agents during the study; had a severe foot infection that required hospitalization; required intravenous antibiotic therapy or additional treatment with non-study antibiotics; required more than 28 days of study drug treatment; or, had received systemic antibiotic therapy for >24 hours within the 72 hours prior to enrollment.
Outcome assessments
Each patient’s clinical condition was assessed by telephone on day 4 ± 1. End-of-treatment (EOT) visit was on day 7 ± 1, day 14 ± 1, day 21 ± 1 or day 28 ± 1, depending on the duration of treatment. Any patient discontinuing therapy early for any reason other than complete cure had to undergo an early termination (ET) assessment within 48 hours after the last dose of study medication. Each patient was also assessed at a test of cure (TOC) visit 12 days (± 2 days) after the EOT or ET visit.
The primary efficacy endpoint was clinical response at the TOC visit, based on evaluating designated clinical signs and symptoms of DFI. We defined clinical success as the total resolution, or improvement of more than two pretreatment clinical signs and symptoms of DFI. We defined clinical failure as persistence or progression of at least one pretreatment clinical sign or symptom after at least 4 days of treatment, or worsening of at least one sign or symptom after discontinuation of therapy in a patient with a previously favorable response. Patients were designated as clinical failure if they need a foot amputation due to failed response after 4 days of treatment, if the patient’s response to therapy was unsatisfactory after at least 4 days of treatment, or if the patient required non-study antibacterial therapy for DFI after at least 4 days of treatment.
Microbiological response was a secondary endpoint and was based on wound culture results from specimens sent to a central microbiology laboratory and clinical response assessments at TOC. Microbiological success was defined as pathogen eradiation (i.e., absence of the original pathogen(s) on a culture of the same site taken at the TOC visit) or presumed eradiation (patient met the definition of clinical success at the TOC visit but a tissue sample could not be obtained for culture from the original infection site). Microbiological failure included persistence (the same organism isolated from the wound on follow-up culture at TOC visit), presumed persistence (clinical failure at TOC visit but tissue sample could not be obtained for culture from the original infection site), superinfection (an organism isolated from the wound that was not present on the initial culture in a patient with clinical signs and/ or symptoms of a wound infection while the patient was on therapy), new infection (isolation of new organism at TOC visit in a patient with clinical signs and/or symptoms of a wound infection), and colonization (a new organism that was not present on the initial culture in a patient without clinical signs and/or symptoms of a wound infection). Patients were also categorized as microbiological failure if they required a foot amputation due to failed response after 4 days of treatment, or if they required non-study antibiotic therapy for DFI after at least 4 days of treatment.
Clinical and microbiological responses were classified as unevaluable if there were circumstances precluding classification in to one of the above responses (e.g., lost to follow up, missing post-treatment information, use of non-study antibacterial therapy for indication other than the DFI, amputation because of reasons other than lack of clinical response of the DFI, less than 4 days of study treatment.).
Endpoints
The primary endpoint was the clinical success rate at the TOC visit in the ITT population. The secondary endpoints were: (1) clinical success rate at the TOC in the PP population and at EOT/ET in the ITT and PP population; (2) microbiological success rate at TOC in the ITT and PP population, and per-pathogen clinical and microbiological success rate.
Wound assessment
A “total wound score” based on IDSA guidelines was compiled by investigators and consisted of combining the general wound parameters (signs and symptoms of infection), and wound measurements (length, width, depth). Each wound parameter was assigned a score based on severity, with higher scores defining greater severity. For wound measurements and undermining, larger measurements received higher scores (Supplementary Information 1). Wounds were also graded by the classification system of IDSA (Supplementary Information 2) [24].
Safety assessment
Safety population included patients who received at least 1 capsule (250 mg) of study drug. Safety and tolerability were evaluated by adverse events (AEs), physical examination, vital signs, electrocardiograms (ECGs), and laboratory tests.
Measurement of nemonoxacin concentrations
Mithra Bioindustry Co., Ltd. (New Taipei City, Taiwan) performed bio analysis of plasma and tissue samples using a solid phase extraction (SPE) method and a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay with a positive multiple reactions monitoring (MRM) detection. High performance liquid chromatography (HPLC) separation was performed on a Luna silica column using a mixture of 0.5% formic acid and methanol/water (55/45; volume/volume) as a mobile phase. Sample concentrations were determined using a weighted linear (1/x) regression of a calibration curve generated from spiked matrix standards. A 200 L aliquot was used for the plasma assay, with a nominal range of quantitation for the analyte of 100 to 5,000 ng/mL. This method was modified from the one used in our Phase 1 study, and demonstrated intra- and inter- day accuracy of 97.81-101.21% and 100.26-102.93%, respectively; precision was within 3%. For soft tissue homogenate analysis, a 100 μL homogenate aliquot was used with a nominal range of quantitation for the analyte of 100 to 5,000 ng/mL. The intra-day accuracy and precision of this method were 96.96-103.94% and 102.61-106.92%, respectively. The analyte in both matrices were stable throughout freeze–thaw cycles, and the bench top and post-operative stability studies.
Pharmacokinetic (PK) and pharmacodynamic (PD) analysis
PK and PD assessments were conducted in a subgroup of four consenting patients on day 7. Blood samples for plasma nemonoxacin concentrations were collected from 4 patients at 0 h, 0.5 h, 1.5 h, 4 h, 6 h, 12 h, and 24 h. Soft tissue samples were collected from 3 of the 4 patients who provided blood samples for nemonoxacin PK analysis. Soft tissue samples (3 mm) were collected by wiping the base of the infected ulcer with dry gauze at 0.5 h, 4 h and 12 h post-dose for 1 of the patients, and 1.5 h, 6 h, and 24 h post-dose for 2 of the patients. Plasma and soft tissue drug concentration-versus-time curves were constructed by using non-compartmental approaches. Noncompartmental pharmacokinetic parameters, including the maximum plasma concentration at steady state (Css,max), time at which maximum plasma concentration was observed (Tmax), terminal elimination halflife (t1/2), area under the concentration-time curve from time 0 to t (AUC0-t), systemic clearance at steady state (CLss/F) and volume of distribution at steady state (Vdss/F) of each subject, were calculated using WinNonlin® software (version 6.2.1, Pharsight Co., CA, USA). The PK/PD parameters fAUC/MIC and fCmax were determined, where f is the unbound fraction of nemonoxacin, using the pharmacokinetic values under steady-state and MIC data for each isolated pathogen.
Statistical analysis
SAS®v Version 8.2 was used for all data processing, summarization, and analyses.
Results
Study population
A total of 38 patients were enrolled into the study, 25 completed the study, and 13 were withdrawn. The reasons for study withdrawal were: consent withdrawn (one); protocol violation (nine); and, adverse event unrelated to the study drug (three). Among those enrolled, 33 patients took at least one whole dose (750 mg) of study medication and had a wound culture at baseline that grew at least one gram-positive pathogen (ITT population); patients from the ITT population who did not have any major protocol violations were included in the PP population (24 patients at EOT/ET and 20 patients at TOC). All 38 enrolled patients received at least one capsule (250 mg) of study medication study medication and were included in the safety population.
Subject demographics and baseline characteristics
Table 1 shows the demographic and clinical characteristics of patients in the ITT population. Most patients were male (66.7%), Caucasian (45.5%), an infection of moderate severity (63.6%), had type 2 diabetes (90.9%), and a baseline HbA1c value between 7.0 and 9.0% (54.5%). Mean age was 59.2 years old.
| Characteristic | Nemonoxacin(N=33) |
|---|---|
| Age, mean years ± SD | 59.2 ± 11.87 |
| Male sex (n, %) | 22 (66.7%) |
| Race (n, %) | |
| Caucasian | 15 (45.5%) |
| African | 7 (21.2%) |
| Asian | 5 (15.2%) |
| Other | 6 (18.2%) |
| Time since diagnosis (mean, years ± SD) | 8.0 ± 6.49 |
| Type 2 diabetes, % of patients | 90.9% |
| HbA1c at entry into the study (n, %) | |
| <6.5% | 6 (18.2%) |
| 6.5% to <7.0% | 2 (6.1%) |
| ≥7.0% to <9.0% | 18 (54.5%) |
| 9.0% to <10.0% | 3 (9.1%) |
| 10.0% to <11.0% | 1 (3.0%) |
| 11.0% to <12.0% | 2 (6.1%) |
| ≥12.0% | 1 (3.0%) |
| Current treatment for diabetes (n, %) | |
| Diet and exercise | 20 (60.6%) |
| Oral agent | 25 (75.8%) |
| Insulin | 16 (48.5%) |
| Other | 0 (0.0%) |
| IDSA infection severity (n, %) | |
| Mild | 12 (36.4%) |
| Moderate | 21 (63.6%) |
| Wound culture | |
| Mean number of culture pathogens perpatient | 2.1 |
| Minimum, maximum number of pathogens/patient Frequency distribution (n, %) with | 1, 6 |
| 1 pathogen | 12 (36.4%) |
| 2 pathogens | 11 (33.3%) |
| 3 pathogens | 6 (18.2%) |
| ≥4pathogens | 4 (12.1%) |
| Medical or surgical history (n, %) | 31 (93.9%) |
| At least one concomitant medication (n, %) | 33 (100%) |
| ITT: intent-to-treat | |
Table 1: Baseline demographic and clinical characteristics of the ITT population.
Based on the results of the Gram-stained smear of the tissue specimens taken at baseline from the infected wound, 26 patients had only gram-positive pathogens and 7 had a mixture of gram-positive and gram-negative pathogens. Culture results showed that the majority (63.6%) of patients had a polymicrobial infection, with a mean of 2.1 isolates per patient.
Almost all (93.9%) of the patients had a history of co-morbid illnesses involving one or more body systems, the most frequent of which were vascular (81.8%) and metabolic or nutritional disorders (48.5%). A total of eight patients reported a prior history of lower extremity amputation, mostly toes. All patients received one or more concomitant medications, the most common of which were drugs for the treatment of diabetes (93.9%).
Clinical and microbiological responses by subject
In the ITT population, the mean duration of treatment with study medication was 10.5 days. Most patients (78.8%) received one (7±1 days) or two (14±1 days) full treatment cycles. Compliance with taking the study drug was 99.6% for the ITT population and 99.4% for the PP population. Table 2 summarized data on the clinical efficacy analysis. Of the 23 patients in the ITT population who were clinically evaluable, 22 were classified as clinical success at TOC, with 14 having complete resolution and 8 improvements in signs and symptoms of infection. Thus, the clinical success rate, which was the primary outcome of interest of this study, was 95.7%. The clinical success rate at TOC for the PP population (94.7%) was similar to that for the ITT population. At the EOT/ET visit, the clinical success rates for both the ITT and PP population were 100%.
| ITT No. of patients (%) |
PP No. of patients (%) |
|||
|---|---|---|---|---|
| TOC | EOT/ET | TOC | EOT/ET | |
| N | 33 | 33 | 20 | 24 |
| Evaluable patients | 23 | 28a | 19 | 23a |
| Clinical success | 22 (66.7) | 28 (84.8) | 18 (90.0) | 23 (95.8) |
| Clinical failure | 1(3.0) | 0 | 1(5.0) | 0 |
| Clinically evaluable | 10 | 5 | 1 | 1 |
| Clinical success rate b | 95.7% | 100% | 94.7% | 100% |
TOC: test-of-cure; ITT: intent-to-treat; EOT: end-of-therapy; ET: early-termination; PP: per-protocol
aThe number of patients at ET visit in the ITT population and PP population was five and three, respectively.
bClinical success rate = 100 x (number of patients with clinical success)/(number of evaluable patients))
Table 2: Clinical response at TOC and EOT/ET in the ITT and PP populations.
The microbiological success rates at TOC for the microbiologically evaluable ITT and PP populations were 82.6% and 89.5%, respectively (see Table 3). Four patients and two patients in the ITT and PP populations had unsuccessful response, respectively. These were due to persistence (presence of at least one of the original pathogens from a repeat culture of the original infection site, 1 patient), presumed persistence (met the definition for clinical failure at TOC visit, but tissue sample cannot be obtained for culture from the original infection site, 2 patients), and colonization (isolation of an organism cultured from an asymptomatic patient, 3 patients).
| No. of patients (%) | ||
|---|---|---|
| ITT | PP | |
| Total patients | 33 | 20 |
| Evaluable patients | 23 | 19 |
| Successful response | 19 (57.6) | 17 (85.0) |
| Unsuccessful response | 4 (12.1) | 2 (10.0) |
| Microbiological success rate a | 82.6% | 89.5% |
TOC: test-of-cure
aMicrobiological success rate = 100 x (number of patients with microbiological success)/(number of evaluable patients))
Table 3: Microbiological response at TOC in the ITT and PP populations.
Clinical and microbiological response by individual pathogen
Table 4 shows the clinical and microbiological responses at TOC for the most frequently isolated pathogens. The most commonly isolated bacteria were gram-positive cocci, with Staphylococcus aureus (found in 69.7%) being the single most frequent isolate. Relatively small numbers of patients had gram-negative organisms isolated from their foot wound. Clinical success rates for each of these five most frequent pathogens were 100% at TOC visit in the ITT population. Microbiological success rates at TOC for gram-positive isolates varied from 75.0% (for Enterococcus faecalis) to 100.0% (for Streptococcus agalactiae and Streptococcus pyogenes). The microbiological success rate for Escherichia coli was lower, at 66.7%. The clinical success rate was 93.8% for polymicrobial infection and 100% for monomicrobial infection.
| Baseline pathogen (No. of isolates) |
Clinical success n/Na (%) |
Microbiological success n/Na (%) |
|---|---|---|
| Staphylococcus aureus (23) | 15/15 (100) | 14/15 (93.3) |
| Streptococcus pyogenes (4) | 4/4 (100) | 4/4 (100) |
| Streptococcus agalactiae (5) | 3/3 (100) | 3/3 (100) |
| Enterococcus faecalis (5) | 4/4 (100) | 3/4 (75) |
| Escherichia coli (7) | 6/6 (100) | 4/6 (66.7) |
| Monomicrobial | 7/7 (100) | 6/7 (85.7) |
| Polymicrobial | 15/16 (93.8) | 13/16 (81.3) |
(TOC: test-of-cure; ITT: intent-to-treat
aUnevaluable patients were excluded)
Table 4: Clinical and microbiological success rates per most frequently isolated baseline pathogens at TOC in the ITT population.
At baseline culture, four patients had MRSA, one of which was quinolone-resistant. The outcome for all four patients was clinical success at EOT and for three of the four the outcome was clinical and microbiological success at TOC. One of these MRSA-infected patients was unevaluable because he had been treated with an antibiotic for another indication between the EOT and TOC time points.
Table 5 shows the MIC ranges of the most frequently isolated pathogens at baseline against nemonoxacin and six other drugs commonly used for DFIs. All of the wound isolates were susceptible to nemonoxacin except three E. coli isolates. Nemonoxacin had a low range of MICs amongst these antibiotics, and specifically had good activity against the S. aureus and E. faecalis isolates.
| MIC range (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Pathogen (n) | Nemonoxacin | Ceftriaxone | Ciprofloxacin | Levofloxacin | Moxifloxacin | Linezolid | Vancomycin |
| S. aureus (23) | 0.03–1 | 2–16 | 0.06–>8 | 0.06–>16 | 0.03–8 | 1–4 | 0.5–1 |
| S. pyogenes (4) | 0.06 | ≤0.03–0.25 | 0.5~1 | 0.5–1 | 0.12–0.25 | 1–2 | 0.25–0.5 |
| S. agalactiae (5) | 0.06–1 | ≤0.03–0.06 | 0.5–>8 | 0.5–>16 | 0.12–4 | 0.25–2 | 0.5 |
| E. faecalis (5) | 0.12–0.5 | >64 | 0.5–>8 | 0.5–16 | 0.25–0.5 | 2 | 1–2 |
| E. coli (7) | 0.12–>16 | ≤0.03–32 | 0.015–>8 | 0.03–16 | 0.06–>16 | >64 | ≥64 |
Table 5: In vitro susceptibility testing results of various antibiotic agents against the most common isolates at baseline.
Wound healing response
The mean change in total wound score from baseline to EOT/ET was -9.6. The improvement was sustained at TOC with a mean change in total wound score from baseline to TOC of -11.2 (Table 6). Similar results were also observed using the IDSA classification. On study entry, the DFI severity was mild in 36.4% of patients and moderate in 63.6%. At the EOT/ET visit, 62.5% of the patients became uninfected, 28.1% had mild infection, and 9.4% had moderate infection. At the TOC, most (82.1%) were uninfected and the severity of infection in all of the remaining five patients was mild. No patient experienced a worsening of IDSA grade from baseline to EOT/ET or TOC.
| N | Mean (SD) | Median | Min, Max | |
|---|---|---|---|---|
| Baseline | 33 | 16.9 (6.30) | 17.0 | 6, 28 |
| Change from Baseline at EOT/ET | 32 | -9.6 (6.35) | -8.5 | -25, -1 |
| Change from Baseline at TOC | 28 | -11.2 (6.91) | -11.0 | -25, 2 |
EOT: end-of-therapy; ET: early termination;TOC: test-of-cure
Table 6: Baseline total wound score and the change from baseline at EOT/ET and TOC in the ITT population.
PK/PD analysis
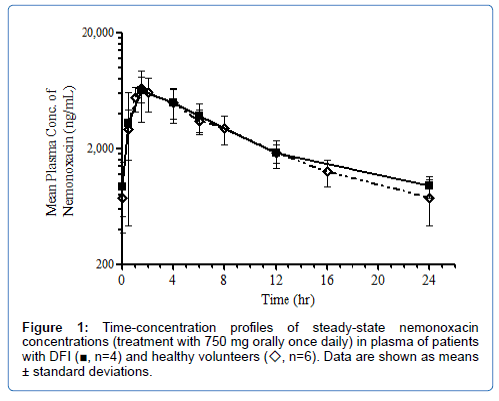
Nemonoxacin was rapidly absorbed following oral administration and Cmax,ss was attained at ~2 hours after dosing. The mean Cmax,ss in plasma was 6,866 ng/mL and the mean AUC0–tau (AUC0-24) was 62,423 ngh/ml (Table 7). The time-concentration profile of nemonoxacin in plasma between the DFI patients of this study and healthy volunteers from a previous phase I study were comparable (Figure 1) [21].
| Parameters | Plasma (mean ± SD) |
|---|---|
| Css, max (ng/mL) | 6,866 ± 2,714 |
| Tmax (h) | 2.13 ± 1.25 |
| t1/2 (h) | 9.15 ± 2.42 |
| AUC0-tau (h*ng/mL) | 62,423 ± 13,462 |
| Vdss/F (mL) | 169,566 ± 73,948 |
| CLss/F (mL/hr) | 12,451 ± 2,700 |
(SD: standard deviation; AUC0-tau: AUC0-24 h (last sampling time point was at 24 h after 7th dose)).
Table 7: Steady-state pharmacokinetic parameters of nemonoxacin in plasma of four patients on the 7th dose.
Because of the limited number of soft tissue samples, the AUC0-24 for soft tissue (192,000 ngh/g) and Cmax,ss in soft tissue (14,600 ng/g) was calculated by combining data from all the collected tissue samples. The degree of tissue penetration of nemonoxacin, determined as a ratio of concentrations in tissue to plasma, were >2.5-fold at most time points (Table 8), and the tissue/plasma ratio of AUC0-24 was 3.08.
| Tissue/plasma ratio | ||||
|---|---|---|---|---|
| Timepoints (h) | Patient 103-001 | Patient 302-005 | Mean of patient 103-001 and 302-005 | Patient 203-003 |
| 0 | ||||
| 0.5 | 1.54 | |||
| 1.5 | 4.64 | 0.92 | 2.78 | |
| 4 | 0.67 | |||
| 6 | 4.68 | 4.97 | 4.83 | |
| 12 | 2.61 | |||
| 24 | 4.01 | 12.83 | 8.42 | |
Table 8: Soft tissue/plasma concentration ratio of nemonoxacin at selected time points after dosing.
To examine the pharmacokinetic-pharmacodynamic correlation, we calculated the AUC/MIC and Cmax /MIC ratios for the bacteria isolated from patients in the PD/PK study before nemonoxacin treatment (Table 9). The fAUC/MICs and fCmax/MICs in plasma were 13.1 to 1747.9 and 1.4 to 192.3, respectively. The AUC/MIC ratios in soft tissue ranged from 48.0 to 3200.0. Among the 4 patients involved in the PK/PD study, 3 achieved microbiological and clinical success at TOC, while 1 was unevaluable due to receiving non-study antibiotic for other medically indicated reasons. However, that subject achieved clinical success at EOT.
| Subject No. | Isolated pathogen | Nemonoxacin MIC (μg/mL) |
Mean plasma fAUC/MIC | Tissue AUC/MIC |
Mean plasma fCmax/MIC |
|---|---|---|---|---|---|
| 103-001 | Streptococcus agalactiae | 1 | 52.4 | 192.0 | 5.8 |
| Streptococcus oralis | 0.25 | 209.7 | 768.0 | 23.1 | |
| Bacteroides fragilis | 4 | 13.1 | 48.0 | 1.4 | |
| Escherichia coli | 0.12 | 437.0 | 1600.0 | 48.0 | |
| 203-003 | Staphylococcus aureus (MRSA) | 0.06 | 873.9 | 3200.0 | 96.1 |
| 302-004 | Staphylococcus aureus | 0.03 | 1747.9 | - | 192.3 |
| Staphylococcus epidermidis | 0.06 | 873.9 | - | 96.1 | |
| Streptococcus pyogenes | 0.06 | 873.9 | - | 96.1 | |
| Streptococcus agalactiae | 0.06 | 873.9 | - | 96.1 | |
| 302-005 | Enterobacter cloacae | 0.5 | 104.8 | 384.0 | 11.5 |
| Enterococcus faecium | 2 | 26.2 | 96.0 | 2.9 |
Table 9: Mean plasma AUC/MIC, Cmax/MIC and combined tissue AUC/MIC of nemonoxacin.
Safety
All enrolled patients received study medication and were included in the safety population. Twenty-one (55.3%) of the patients reported AEs, and 7 (18.4%) reported drug-related AEs. Drug-related AEs reported were diarrhea and headache (2 patients each); constipation, pruritus, swelling of face, electrocardiogram QT prolonged, and epistaxis (1 patient each). AEs were mostly mild to moderate. Five subjects reported 7 SAEs that were considered not related to study medication by the investigator. Four patients experienced AEs that led to study drug discontinuation, 3 of which were SAEs: amputation due to osteomyelitis (1 patient), amputation due to gangrene (1 patient), and ectomy due to abscess (1 patient). The other AE that led to study drug discontinuation was decreased creatinine clearance of mild intensity that was considered unlikely to be related to study medication. No deaths occurred during the study, and there was no clinically significant change in mean values from baseline for any of the laboratory parameters or vital signs.
Discussion
Treatment of diabetic foot infections requires antibiotic therapy, as well as proper wound care. Most mild to moderate infections in patients without gastrointestinal absorptions problems can be treated with an oral antibiotic that has an appropriate antimicrobial spectrum [24]. Nemonoxacin, a broad-spectrum oral antimicrobial agent with especially good activity against gram-positive cocci, including MRSA [15-17] could be a potentially useful drug for these commonly polymicrobial infections. This study is the first to evaluate nemonoxacin in patients with diabetic foot infections. Results showed that in clinically evaluable patients, the clinical success rates at TOC for ITT and PP populations were high (95.7% and 94.7%, respectively), as were the microbiological success rates (82.6% and 89.5%, respectively).
Nemonoxacin also demonstrated a good safety profile. Drugrelated adverse events were reported by 18.4% of the patients, with gastrointestinal (7.9%) and nervous system disorders (5.3%) being the most common. These results are similar to those reported in previous nemonoxacin trials [18,20]. Four patients experienced an AE that led to discontinuation from the study, three of which were classified as SAEs, but none were considered drug-related. These adverse event rates are similar to those reported in studies of patients with DFI treated with fluoroquinolones [25-27].
For successful clinical outcomes the administered antibiotic must attain therapeutic concentrations in the infected tissues. Penetration of antimicrobial agents into foot tissue in persons with diabetes is often inadequate due to peripheral vascular disease involving larger vessels, as well as micro vascular and capillary disease [24,28,29]. Another factor that may affect the tissue penetration and volume of distribution of an antibiotic is its plasma protein binding [30]. The low protein binding rate (approximately 16%) of nemonoxacin may account for its high concentration in tissues in this study [31]. The mean tissue/plasma ratios of orally administrated moxifloxacin and levofloxacin are 1.01±0.57 and >1.0, respectively [28,31]. In this study, the concentrations of nemonoxacin in tissues were 2.5 fold higher than plasma in almost all sampling time points for the 4 subjects who participated in the PK study. These results suggested that nemonoxacin could penetrate well and achieve high concentrations in wound tissues of diabetic foot ulcers. However, it should be noted that the number of tissue samples are very small and there was a large individual variability of nemonoxacin PKs in the tissues.
The results of this study correlate well with the in vitro studies of activity of nemonoxacin against common pathogens found in DFI [15-17]. Nemonoxacin had high microbiological success rates (75%-100%) against S. aureus (including 3 MRSA isolates), S. pyogenes, S. agalactiae, and E. faecalis. While the microbiological success rates against E. coli were lower (66.7%), the clinical success rates for patient from whom this pathogen was isolated was 100%. AUC/MIC ratios of 100 to 125 or Cmax/MIC ratios of >10 are predictive of clinical and microbiological success with limited development of bacterial resistance [32,33]. The patients who participated in the PK study had high ratios of AUC/MIC and Cmax/MIC for almost all pathogens in both the plasma and tissue. The AUC/MIC of the tissue was 3.7 fold higher than plasma for all the pathogens tested. In one patient with MRSA, who became unevaluable at TOC, the AUC/MIC were very high (873.9 mean plasma and 3200 in tissue).
Conclusion
In summary, in this small non-comparative prospective trial, treatment with nemonoxacin was well tolerated by patients with DFI and was associated with high clinical cure and microbiological success rates. Nemonoxacin demonstrated high activity against the common causative pathogens and good pharmacokinetic and pharmacodynamic properties. These results suggest that once-daily oral administration of nemonoxacin may be appropriate for treating mild to moderate DFI. Due to the limited number of subjects in this study, further comparative studies in larger patients groups are required to explore the role of nemonoxacin against other approved agents in the therapy of patients with DFI.
References
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J (2005) The global burden of diabetic foot disease. Lancet 366:1719-1724.
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, et al. (2018) IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2017 and projections for 2045. Diabetes Res ClinPract 138:271-281.
- Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376:2367-2375.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, et al. (2007) High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia 50:18-25.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, et al. (2012) 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 54: e132-e173.
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ (2003) Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 26:1435-1438.
- Citron DM, Goldstein EJ, Merriam CV, Lipsky BA, Abramson MA (2007) Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J ClinMicrobiol 45:2819-2828.
- Frykberg RG (2003) An evidence-based approach to diabetic foot infections. Am J Surg 186:44S-54S.
- Yates C, May K, Hale T, Allard B, Rowlings N, et al. (2009) Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care 32:1907-1909.
- Dang CN, Prasad YD, Boulton AJ, Jude EB (2003) Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med 20:159-161.
- Galkowska H, Podbielska A, Olszewski WL, Stelmach E, Luczak M., et al. (2009) Epidemiology and prevalence of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis in patients with diabetic foot ulcers: focus on the differences between species isolated from individuals with ischemic vs. neuropathic foot ulcers. Diabetes Res ClinPract 84:187-193.
- Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, et al. (2010) Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia 53:914-923.
- Eleftheriadou I, Tentolouris N, Argiana V, Jude E, Boulton AJ (2010) Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs 70:1785-1797.
- Murugan S, Mani KR, Devi PU (2008) Prevalence of methicillin resistant Staphylococcus aureus among diabetes patients with foot ulcers and their antimicrobial susceptibility pattern. J ClinDiagn Res 2:979-984.
- Lauderdale TL, Shiau YR, Lai JF, Chen HC, King CHR (2010) Comparative in vitro activities of nemonoxacin, (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrob Agents Chemother 54:1338-1342.
- Chen YH, Liu CY, Lu JJ, King CHR, Hsueh PR (2009) In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci, and Streptococcus pneumoniae with various resistant phenotypes in Taiwan. J AntimicrobChemother 64:1226-1229.
- Chen YH, Liu CY, Ko WC, Liao CH, Lu PL, et al. (2014) Trends in the susceptibility of methicillin-resistant Staphylococcus aureus to nine antimicrobial agents, including ceftobiprole, nemonoxacin, and tyrothricin: results from the TigecyclineIn Vitro Surveillance in Taiwan (TIST) study, 2006-2010. Eur J ClinMicrobiol Infect Dis 33:233-239.
- vanRensburg DJJ, Perng RP, Mitha IH, Bester AJ, Kasumba J, et al. (2010) Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob Agents Chemother 54:4098-4106.
- Liu Y, Zhang Y, Wu J, Zhu D, Sun S, et al. (2017) A randomized, double-blind, multicenter phase II study comparing the efficacy and safety of oral nemonoxacin with oral levofloxacin in the treatment of community-acquired pneumonia. J MicrobiolImmunol Infect 50:811-820.
- Yuan J, Mo B, Ma Z, Lv Y, Cheng S-L, et al. (2017) Safety and efficacy of oral nemonoxacin versus levofloxacin in treatment of community-acquired pneumonia: a phase 3, multicenter, randomized, double-blind, double-dummy, active-controlled, non-inferiority trial. J MicrobiolImmunol Infect 52(1):35-44.
- Chung DT, Tsai CY, Chen SJ, Chang LW, King CHR, et al. (2010) Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob Agents Chemother 54:411-417.
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, et al. (2006) Diagnosis and treatment of diabetic foot infections. PlastReconstrSurg 117:212S-238S.
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, et al. (2004) Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 39:885-910.
- Graham DR, Talan DA, Nichols RL, Lucasti C, Corrado M, et al. (2002) Once-daily, high-dose levofloxacin versus ticarcillin-clavulanate alone or followed by amoxicillin-clavulanate for complicated skin and skin-structure infections: a randomized, open-label trial. Clin Infect Dis 35:381-389.
- Lipsky BA, Miller B, Schwartz R, Henry DC, Nolan T, et al. (1999) Sparfloxacin versus ciprofloxacin for the treatment of community-acquired, complicated skin and skin-structure infections. ClinTher 21:675-690.
- Vick-Fragoso R, Hernández-Oliva G, Cruz-Alcázar J, Amábile-Cuevas CF, Arvis P, et al. (2009) Efficacy and safety of sequential intravenous/oral moxifloxacinvs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection 37:407-417.
- Oberdorfer K, Swoboda S, Hamann A, Baertsch U, Kusterer K, et al. (2004) Tissue and serum levofloxacin concentrations in diabetic foot infection patients. J AntimicrobChemother 54: 836-839.
- Majcher-Peszynska J, Haase G, Sass M, Mundkowski R, Pietsch A, et al. (2008) Pharmacokinetics and penetration of linezolid into inflamed softtissue in diabetic foot infections. Eur J ClinPharmacol 64: 1093-1100.
- Barza M (1993) Anatomical barriers for antimicrobial agents. Eur J ClinMicrobiol Infect Dis 1:S31-35.
- Lin L, Chang LW, Tsai CY, Hsu CH, Chung DT, et al. (2010) Dose escalation study of the safety, tolerability, and pharmacokinetics of nemonoxacin (TG-873870), a novel potent broad-spectrum nonfluorinated quinolone, in healthy volunteers. Antimicrob Agents Chemother 54:405-410.
- Majcher-Peszynska J, Sass M, Schipper S, Czaika V, Gussmann A, et al. (2011) Pharmacokinetics and penetration of moxifloxacin into infected diabetic foot tissue in a large diabetic patient cohort. Eur J ClinPharmacol 67:135-142.
- Stass H, Dalhoff A (2005) The integrated use of pharmacokinetic and pharmacodynamic models for the definition of breakpoints. Infection 2:29-35.
- Wright DH, B rown GH, Peterson ML, Rotschafer JC (2000) Application of fluoroquinolone pharmacodynamics. J AntimicrobChemother 46:669-683.
Citation: Lipsky BA, Ganib M, Rogers LC, Hwang JS, Tsaie CY, et al. (2019) A Pilot Study of Nemonoxacin in Patients with Diabetic Foot Infections. J Infect Dis Ther 7: 397. DOI: 10.4172/2332-0877.1000397
Copyright: © 2019 Lipsky BA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3412
- [From(publication date): 0-2019 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 2593
- PDF downloads: 819