Research Article Open Access
A Pilot Study - Comparison between a Novel Combination of Bioactive Glass with Clodronate and Bioactive Glass Alone as a Treatment for Chronic Periodontitis
Kirsi Rosenqvist1*, Mervi Gürsoy2,3#, Eija Könönen2,3, Ulvi K Gürsoy2 and Anne M Juppo11Division of Pharmaceutical Chemistry and Technology, Formulation and Industrial Pharmacy Unit, Faculty of Pharmacy, University of Helsinki, Viikinkaari, Finland
2Department of Periodontology, Institute of Dentistry, Lemminkäisenkatu 2, University of Turku, Finland
3Oral Health Care, Welfare Division, City of Turku, FI20101 Turku, Finland
4Author contributed equally to this work
- Corresponding Author:
- Rosenqvist K
Division of Pharmaceutical Chemistry and Technology
Formulation and Industrial Pharmacy Unit
Faculty of Pharmacy, University of Helsinki
Viikinkaari 5 (P.O. Box 56), 00014, Finland
Tel: +358405124993
Fax: +358919159144
E-mail: kirsi.rosenqvist@helsinki.fi
Received date: June 29, 2017; Accepted date: July 19, 2017; Published date: July 26, 2017
Citation: Rosenqvist K, Gürsoy M, Könönen E, Gürsoy UK, Juppo AM (2017) A Pilot Study - Comparison between a Novel Combination of Bioactive Glass with Clodronate and Bioactive Glass Alone as a Treatment for Chronic Periodontitis. J Biotechnol Biomater 7:265. doi:10.4172/2155-952X.1000265
Copyright: © 2017 Rosenqvist K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Bioactive glass (BAG) and clodronate are both used for bone regeneration. In this pilot clinical study, we compared the effect of BAG and a novel BAG+clodronate combination as a topical maintenance phase treatment for chronic periodontitis. Two dental residual pockets were treated in each subject (n=10): one with BAG alone and the other with combination product, by applying the products subgingivally for 10 min once a week for four weeks. We describe the effects of these investigational products to the clinical parameters of periodontitis and two bone metabolism markers (osteoprotegerin and osteocalcin). Additionally, subjective satisfaction for the treatment was evaluated. The results must be considered as directional, understanding that further investigation is needed to confirm the findings. Based on clinical parameters measured both treatments could benefit as maintenance therapy for chronic periodontitis. The positive effect of the combination product on tooth sensitivity may bring additional benefits in comparison to the use of BAG alone. Both treatments were well tolerated and safe.
Keywords
Bioactive glass; Clodronate; Periodontitis
Abbreviations
BAG: Bioactive Glass; BAG+C: Combination of Bioactive Glass and Clodronate; BOP: Bleeding on Probing; COX-2: Cyclooxygenase-2; F: Follow-Up Visit; GCF: Gingival Crevicular Fluid: min: Minute; OPG: Osteoprotegerin; OC: Osteocalcin; PAL: Probing Attachment Level; PPD: Probing Pocket Depth: TV: Treatment Visit; VAS: Visual Analog Pain Scale; VPI: Visible Plaque Index
Introduction
Periodontitis is a reversible condition that, if left untreated, may develop into chronic periodontitis, as plaque induced gingivitis extends from the gingiva and leads to irreversible progressive loss of alveolar bone and periodontal ligament attachment. Ultimately, periodontitis can cause tooth loss. In periodontal disease the height of alveolar bone is lower which results in deeper pockets [1]. The aim of periodontitis therapy is to reduce gingival inflammation, reduce pocket depth as well as bone loss and increase attachment to gingiva. Usually this can be accomplished by non-surgical means with mechanical removal of the bacterial biofilm and their toxins from the tooth surface making it compatible with biologic reattachment that is the basis of any eventual adjunctive therapy. Both surgical and non-surgical periodontal treatments result in recession of the gingival margin after healing. Localized recession and root exposure is often associated with dentin hypersensitivity [1-4]. The maintenance phase of periodontal therapy is defined as the maintenance of periodontal health following active, primary treatment of periodontitis in order to achieve long-term stability of results and to minimize recurrence. Currently there is no established medical treatment for periodontitis maintenance phase, which would help in reducing pocket depth as well as bone loss and increase attachment to gingiva.
Bioactive glass (BAG, Na2O-CaO-P2O5-SiO2) has been used for long time as a bone cavity-filling material in the cranio-maxillofacial area (e.g. frontal sinus obliteration after severe chronic sinusitis), in orthopedics, and in the treatment of osteomyelitis [5-7]. During the last two decades, its use has expanded into dentistry, for instance, for treating cortical bone defects [8] and hypersensitive teeth [4,9]. There are obvious similarities between bone and dentine concerning their metabolism. BAG is potentially useful as a means of stimulating remineralization of the upper layer of the tooth and protecting the tooth against alterations of temperature, pressure, solute concentrations, and local irritation [4,9]. BAG is known to have bacterial growth-inhibiting properties [7,10] and this could provide even more advantages in the treatment of periodontitis, i.e., infection-induced inflammationregulated degradation of tooth-supporting tissues. Moreover, the combination of bisphosphonates with bioactive glass provides protection against biofilms formed by the periodontal pathogen Aggregatibacter actinomycetemcomitans [11].
Clodronate is a bisphosphonate and although intravenously administered systemic bisphosphonate therapy can have a negative side effect, namely osteonecrosis of the mandibule [12], systemically administered bisphosphonate therapy seems to have beneficial effects on the periodontium: less plaque accumulation, gingival inflammation and periodontal attachment loss, lower probing depths, and greater alveolar bone levels [13]. Additionally, there is information available suggesting bone formation when using the combination of BAG and bisphosphonate administered locally in surgery as a filling material of bone defects [14,15].
The activity of cells involved in bone turnover can be assessed using chemical biomarker measurements from body fluids. This can be useful to determine the effectiveness of the treatment. There are several biomarkers that describe bone turnover and they can be divided into those indicating bone resorption or formation. Osteoprotegerin (OPG) is a good biomarker for bone resorption and bone loss [16] and osteocalcin (OC), in turn, indicates bone formation [17]. In the present study, these two biomarkers were chosen to be measured from gingival crevicular fluid (GCF).
Recently, it has been shown that clodronate enhances the bioactivity of BAG and keeps this effect ongoing longer than what is observed for BAG alone [18]. Based on experiment done by Rosenqvist and coworkers [18], it is apparent that there is a strong interaction between BAG and clodronate resulting in an enhanced ion exchange. The interaction seems to be such that an extended ion exchange between the BAG and clodronate results in a layer of calcium clodronate on the surface of the bioactive glass. The formed layer includes also calcium phosphate [19]. Most importantly, the understanding is that clodronate enhances the bioactivity of bioactive glass and both calcium clodronate precipitation and apatite are formed. Therefore, the combination of BAG and clodronate (BAG+C) could have the potential to support locally the bone formation and be beneficial also in periodontal applications and be established treatment for periodontitis maintenance phase, which we currently lack. So far, no published data exist on the topical use of clodronate in the treatment of periodontal disease or on the use of the BAG+C combination as a clinical application. Within this background, the aim of the present preliminary pilot study was to investigate the effects of the combination product, namely BAG+C, on periodontal residual pockets and to compare the results on those of BAG alone. In order to investigate this, the experiment included measurements of clinical parameters, representing signs and symptoms of periodontitis, and bone metabolism markers in GCF. In addition, subjective assessment of tooth function and overall satisfaction for the treatment were recorded during the study.
Materials and Methods
Study conduct and ethics
This pilot, open single-centre, comparative clinical investigation was conducted at the Institute of Dentistry, University of Turku, Finland, according to the clinical investigation plan, International Organization for Standardization: Clinical investigation of medical devices for human subjects-Good clinical practice (ISO 14155), European Union Directive: Good Clinical Practice and Helsinki Declaration. The ethical approval was obtained from the ethics committee of the Hospital District of Southwest Finland and the study was reported to the National Supervisory Authority for Welfare and Health in Finland.
Investigational study products: Bioactive glass (BAG) and clodronate
Bioactive glass (S53P4), BonAlive™, is a CE-marked product (medical device with marketing authorization in EU) by the BonAlive Biomaterials Ltd, Turku, Finland. The BonAlive™ product contains four oxides, SiO2 53%, Na2O 23%, CaO 20%, and P2O5 4% w/w. It is composed of amorphous odourless white granules with a particle size of 0.5-0.8 mm, having the density of 2.66 g/cm3. Clodronate (Lot No T07/009 Ph. Eur.) was supplied by PharmaZell GmbH, Raubling, Germany. It was in the form of disodium salt (CH2Cl2O6P2Na2 4H2O 360.9 g/mol).
Based on pre-formulation studies the combination of 200 mg clodronate and 1 g BAG with particle size 0.5-0.8 mm was proven as the most promising formulation for dental application to treat periodontitis [19]. Therefore, this was chosen as the test product, BAG+C, while the comparator product was 1 g BAG, both with 900 μl of 0.9% saline added. The final, paste-like, preparation of the study products was done during the same day as the products were applied into the selected periodontal pockets at the study clinic.
Study design and subjects
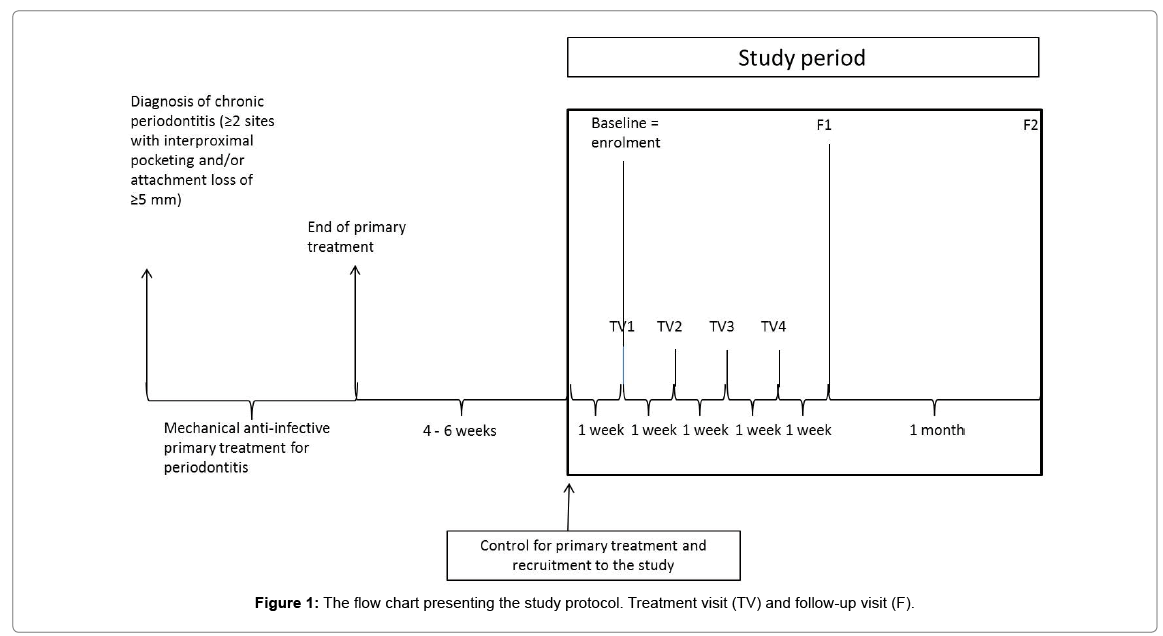
A total of 10 eligible, consenting study subjects, either men or women, were recruited to the investigation. According to the inclusion criteria, a subject had a diagnosis of chronic periodontitis as defined by at least two sites with probing pocket depth (PPD) ≥ 5 mm interproximally and had received conventional, anti-infective, mechanical treatment for the disease, but still had at least two residual periodontal pockets. The exclusion criteria were as follows: concurrent disease or condition that, in the opinion of the investigator, was contraindicating participation, simultaneous participation in another medical device or investigational drug trial, systemic antibiotic treatment, and smoking. According to the study protocol that is illustrated in Figure 1, the periodontal status was re-evaluated 4-6 weeks after mechanical, primary treatment. Two residual pockets with PPD of 4-6 mm, involving an identical tooth type and site, were selected for investigational product application. Eligible study subjects were provided first with a detailed information letter. One week later, at the enrolment visit, the volunteers gave their written consent. Additionally, baseline data and measures, including GCF sampling from two selected tooth sites, were performed before the first treatment (BAG+C and BAG) application. The study included a total of four treatment visits (TV1-TV4); once per subsequent week, BAG+C was applied into one residual pocket and BAG into the other pocket for 10 min and left subgingivally. In this way, the subjects acted as their own comparator and control. In addition, the study included two follow-up visits; the first follow-up visit (F1) occurred one week and the second visit (F2) five weeks after the last treatment visit (=TV4, the last product application). Thus, the duration of the study was about two months for a single participant.
Outcome measures
At the enrolment visit, the following data were recorded: age, gender, ethnic origin, and body mass index, information on relevant previous and current disease (medical history), smoking history, and alcohol consumption. Throughout the investigation, periodontal status at the tested sites was recorded comprising the following clinical parameters: visible plaque index (VPI), bleeding on probing (BOP) dichotomously (present/absent), PPD (probing pocket depth i.e., the distance between the gingival margin and the bottom of the pocket), gingival recession (the distance between the cemento-enamel junction and the gingival margin) and probing attachment level (PAL, i.e., the amount of attachment loss; derived from PPD and gingival recession) in millimeters measured with a WHO probe. Furthermore, at each visit, tooth sensitivity to air blow and cold water spray was examined and recorded by using visual analog pain scale (VAS; 0=no pain, 10=worst pain). From TV2 onwards, the participants were asked to evaluate possible changes (better, unchanged, or worse) in tooth function (subjective sensation and treatment success) with the treated sites compared to the situation at their previous visit and to appraise current overall satisfaction with the scale: excellent (1), good (2), satisfactory (3), or poor (4). As a safety variable, adverse events and device effects were collected starting from the screening and throughout the investigation. Additionally, all concomitant medications and treatments with the reason to administer were recorded during all visits.
GCF sampling, biomarker and data analysis
GCF samples were collected three times, at baseline and during FI and F2. After removing supragingival plaque and avoiding any saliva contamination, GCF sampling was performed by placing a paper strip into the selected residual pocket for 30 s. Any paper strip containing blood was discarded. The samples were placed into the Eppendorf tubes, which were stored at -20°C until assayed. The GCF samples were analyzed for two bone remodeling biomarkers, OPG and OC, by using a commercially available kit (Milliplex HBN1A-51K Osteoprotegerin/ Osteocalcin, Merck Millipore, St. Charles, Missouri, USA) according to the manufacturer’s instructions. The primary analysis population was a full analysis set, which included all study subjects. For the statistical analyses within the group, the 2-tailed Wilcoxon signed ranks tests and between two groups the Mann-Whitney U test were used. No imputation procedures were applied on missing data. The statistical significance was set at p value<0.05. Because the number of samples/data is limited, it needs to take notice of that, even the statistical analyses were performed, the result can be considered only as directional.
Results
Study subjects
Eight out of the 10 study subjects were men. The mean age of participants (all Caucasian) was 66.5 years and their mean body mass index was 26. Six subjects were former smokers. Two subjects had medication for hypercholesteremia and hypothyreosis; one had medication for prostate hyperplasia, one for hyperactive urinary bladder, and one for menopausal symptoms, while five subjects did not use any ongoing medications. Seven subjects participated at all scheduled visits and eight completed all follow-up visits. One subject discontinued prematurely after TV1 because of an adverse event (Figure 1). One subject was unable to attend a treatment visit and the other one a follow-up visit.
Clinical parameters
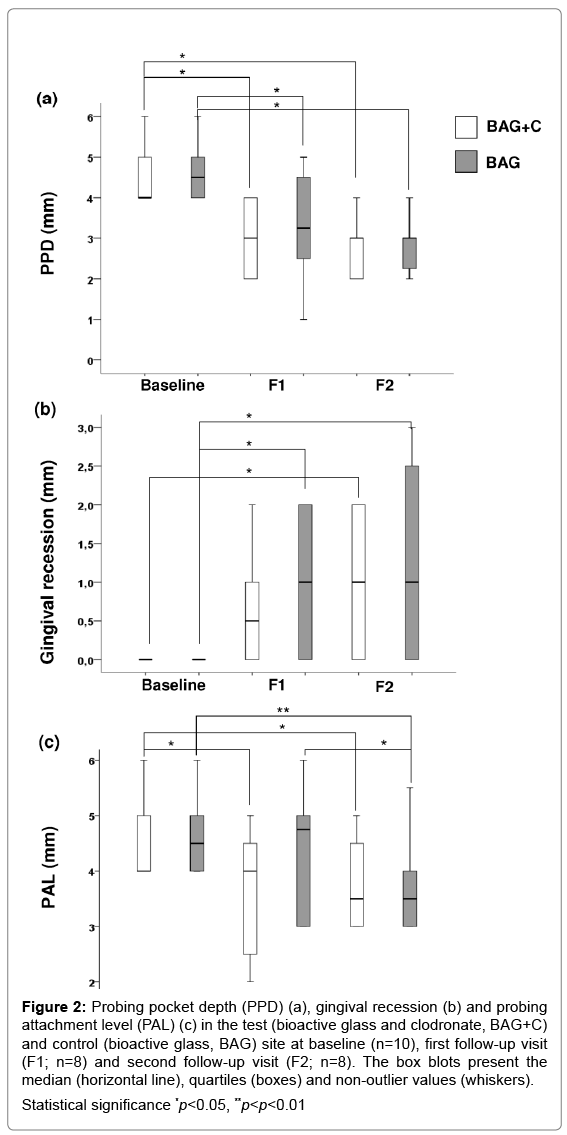
PPD values reduced significantly in BAG+C and BAG treated teeth during the study (Figure 2a), while no differences in VPI or BOP values were observed. Gingival recession (the apical movement of the gingival tissue boundary) increased significantly for both treatment options from the baseline to F2 (Figure 2b). PAL decreased significantly in BAG+C treated teeth at both F1 and F2 as compared to the baseline (Figure 2c), whereas in BAG treated teeth the significant result was seen only at F2.
Figure 2: Probing pocket depth (PPD) (a), gingival recession (b) and probing
attachment level (PAL) (c) in the test (bioactive glass and clodronate, BAG+C)
and control (bioactive glass, BAG) site at baseline (n=10), first follow-up visit
(F1; n=8) and second follow-up visit (F2; n=8). The box blots present the
median (horizontal line), quartiles (boxes) and non-outlier values (whiskers).
Statistical significance *p<0.05, **p<p<0.01
Biomarkers
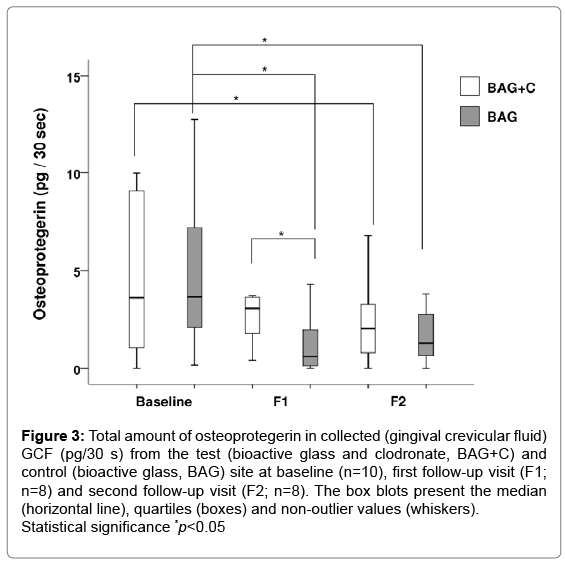
There was a significant change in the amount of OPG in BAG treated teeth at both follow-up visits when compared to the baseline value (Figure 3), while in BAG+C teeth there was a significant reduction in the amount of OPG only between the baseline and F2. A significant difference was observed between the treatment options at F1 when teeth treated with BAG had lower OPG levels than those treated with BAG+C. OC values remained at low levels throughout the study in both treatment options.
Figure 3: Total amount of osteoprotegerin in collected (gingival crevicular fluid) GCF (pg/30 s) from the test (bioactive glass and clodronate, BAG+C) and control (bioactive glass, BAG) site at baseline (n=10), first follow-up visit (F1; n=8) and second follow-up visit (F2; n=8). The box blots present the median (horizontal line), quartiles (boxes) and non-outlier values (whiskers). Statistical significance *p<0.05
Subjective sensation, treatment success and safety
Starting from TV2, the subjects were asked for change in the tooth function (better, unchanged, or worse) since the previous visit. There were no differences between the treatment options up to F2. There was one subject who estimated BAG+C better and one subject vice and versa, while other subjects considered both treatment options equal (the median value was “unchanged”). At F2, the subjects reported the tooth function to be slightly better in teeth treated with BAG+C (median: BAG+C better vs. BAG unchanged). Concerning hypersensitivity of the treated teeth, tested by air blow and cold water, all subjects estimated the pain to be less for BAG+C treated teeth than for BAG alone at F2.
At each visit, the mean value for overall satisfaction of treatment was reported slightly better for BAG+C treated teeth, although the median value was similar for both treatment options (Table 1). During the both follow-up visits, the overall satisfaction for BAG+C treated teeth varied from excellent to good and for BAG treated teeth from excellent to satisfactory (F1) or even poor (F2).
| Visit | BAG+C | BAG | ||
|---|---|---|---|---|
| Mean | Median (min-max) | Mean | Median (min-max) | |
| TV2 (n=10) | 2.4 | 2 (2-4) | 2.5 | 2 (2-4) |
| TV3 (n=9) | 2.11 | 2 (2-3) | 2.33 | 2 (2-4) |
| TV4 (n=8) | 2 | 2 (1-3) | 2.25 | 2 (1-4) |
| F1 (n=8) | 1.88 | 2 (1-2) | 2 | 2 (1-3) |
| F2 (n=8) | 1.88 | 2 (1-2) | 2.13 | 2 (1-4) |
| Total (n=8) | 2.05 | 2 (1-4) | 2.24 | 2 (1-4) |
Table 1: Mean and median score for study subject’s (n=number of subjects) evaluation for the situation (excellent (1), good (2), satisfactory (3), poor (4)) compared to that at previous visit in teeth treated with either combination product of bioactive glass and clodronate (BAG+C) or bioactive glass (BAG) alone (TV=treatment visit; F=follow-up visit).
There was only one adverse event recorded during the study; one subject had a mucosal lesion at the gingival area of a BAG+C treated tooth after the first application that led to the premature termination of the study. The situation was recovered within a month. Otherwise both treatment options were well tolerated and there were no safety issues reported.
Discussion
The novel combination product, BAG+C, was tested in a clinical pilot study, where its effects were compared to those of BAG alone at periodontal maintenance phase, without compromising the safety of the subjects. Since both BAG and clodronate separately are known to have beneficial effects to bone (e.g. stimulating the bone formation), there is a reason to believe that their synergistic effect is even better. BAG is known to be safe in clinical use, however, no combination product with bisphosphonates and BAG has been previously studied. Moreover, there is no published investigation about bisphosphonates used in the local treatment of deepened pockets at periodontal maintenance phase. Although our findings did not differ significantly between the given treatment options in general, looking at the investigated variables together, like PPD, OPG, change in tooth function, pain sensation after cold water and air blow, as well as overall satisfaction for treatment, the BAG+C product appeared to have a little better results than BAG alone. Indeed, the slightly better results in variables, especially the subject´s own feeling, indicate that adding clodronate to BAG may enhance the already known favorable effects of BAG alone.
Based on published data, it is well defined that BAG has several beneficial effects in dental use [8-10]. In addition, it also improves the outcome in the given indication, i.e., periodontitis maintenance phase. This includes the advantageous effects of BAG on periodontal clinical signs, such as decreased PPD and increased clinical attachment level. These studies were done with an appropriate control arm [20,21]. A recent meta-analysis, summarizing results from randomized controlled clinical trials on the effect of BAG as a graft material in the treatment of periodontal intrabony defects, supports this information [22]. Indeed, BAG proved to be superior compared with other graft materials as well as with open flap debridement, seen as a considerable improvement in PPD and clinical attachment levels. Therefore, in the present study, the teeth treated with BAG alone served as a positive control group for the teeth treated with the test combination product BAG+C. Our study also proved BAG to be useful for the treatment of residual pockets at periodontal maintenance phase.
In the current literature, there are several animal studies on the potential of using bisphosphonates for the management of periodontitis or preventing root resorption when moving teeth by orthodontic means. In an experimental study on periodontitis in rats, Mitsuta et al. [23] suggested that the local administration of clodronate may be effective in preventing osteoclastic activity leading to bone resorption, which is the main characteristic of periodontitis. Another bisphosphonate agent, alendronate, was examined in a beagle dog model for its capability of inhibiting alveolar bone loss [24]. In the alendronate group, a significant increase in bone mass was observed compared to placebo groups. In a rat study by Yaffe et al. [25], where local delivery of alendronate was used together with tetracyclines, a combined effect was demonstrated in the reduction of alveolar bone loss. Furthermore, as a potent blocker of bone resorption, risedronate has been examined in the context of orthodontic tooth movements in rats [26]; it was suggested that the local administration of risedronate has favorable effects by preventing root resorption.
There are some published data available about the use of combination of bisphosphonate and BAG as a local treatment. Välimäki et al. [14] examined the effect of subcutaneously administered zoledronic acid and BAG as a bone graft substitute in rat tibia. They conclude that the beneficial effect of bisphosphonate therapy may extend to healing of implants with bioactive hydroxyapatite coatings. In another case, where the combination of alendronate and BAG was studied in the rat mandible as a filling material of a created bone defect, the combination proved to induce bone regeneration and was thus considered to be useful for alveolar ridge augmentation followed by dental implant surgery and for bone regeneration in periodontal defects [15].
Of the two bone remodeling biomarkers selected for the present study, OPG data indicate that the effect of the combination product (BAG+C) is slightly better than the effect of BAG alone. However, due to the short time period of investigation as well as the limited number of subjects and treated teeth, the result remains only indicative. OPG is known to inhibit the differentiation activity of osteoclasts (bone resorbing cells) by blocking ligand RANK (RANKL, receptor activator of nuclear factor-kappa B for osteoclast precursor cell), which is a good biomarker for bone resorption and bone loss [16]. Bisphosphonates cause osteoblasts (bone forming cells) to release OPG [27]. Clodronate is known to inhibit osteoclast activity through apoptosis [28] and, further, COX-2 dependent prostaglandin E2 production and RANKL expression in periodontal ligament cells [29]. The biomarker needs to clearly reflect the variable of interest, e.g. bone resorption and be specific and sensitive enough. In these published studies listed above, the release of OPG was examined from sites with nitrogen containing bisphosphonates, the possible effect for decreased bone degradation may not be seen so clearly in our investigation using OPG as a biomarker as clodronate is not nitrogen-containing bisphosphonate. On the other hand, decreased OPG levels could indicate that bone turnover was activated, because in order to have bone formation there is need to have also resorption. However, in that case the bone formation marker OC should have demonstrated more activity. Bone metabolism processes are slow and do not happen in weeks. Therefore, it might be that our biomarker results describe the early phase of the treatment success and the later part was still to come. Although it is known that BAG releases ionic products and enhances the expression of osteogenic markers like OC [17], we failed to demonstrate any change at OC levels. In a way, this could be a good sign, if interpreted that neither of the investigated treatments decreased the activity of osteoblasts as such. Both OPG and OC are regulated and effect mediated via osteoblasts [16,17]. As clodronate mostly has an influence on osteoclasts and BAG on osteoblasts, it might be that both selected biomarkers reflect mostly the effect of BAG, not that of BAG+C, i.e., the benefit of having clodronate in the combination product. This could explain why OC levels did not differ between the products and the change in OPG levels was only minor. Therefore, in future studies also other bone turnover biomarkers, such as osteopontin, osteonectin and bone sialoprotein, should be considered. Moreover, radiological examination after a longer follow-up period than two months could give more information about the quality of bone. In our pilot study, the patients were protected from exposure to radiation.
In the present study, the subjects were asked for their opinion about the tooth function and hypersensitivity in the treatment area, and overall satisfaction. The outcome in BAG+C treated teeth was better than that in BAG treated teeth. Based on results of clinical signs and biomarkers, both treatment options can be considered equally good. Copay et al. [30] reviewed the dilemma, whether the difference in clinical parameters or the subjective outcome is more important from the patient’s point of view. They conclude that, even though the general concept for this is lacking, the self-reported outcomes should have more value and be distinguished from objective clinical measures. Therefore, with caution, we suggest that the subject’s self-reported results can be considered to verify that BAG+C is slightly better than BAG alone in the treatment of residual pockets at periodontal maintenance phase.
The limitations of our study include the limited number of participants, rather few applications of test and control agents, and a relatively short follow-up period. Compared to earlier investigations done with BAG, for example, Biosilicate® (fully crystallized bioactive glass) in the treatment of hypersensitive teeth [9], the treatment was given twice daily for 30 days. Therefore, it was surprising that with this limited amount of applications, our study gave such good results. In addition, reduced pain scores were similar to those observed in the study by Tirapelli et al. [9] and the reduction in pain remained up to the completion of follow-up visits. The study duration and number of applications of tested agents were kept in minimum, because the combination product (BAG+C) was used for the first time in a clinical setting.
The only adverse event in the present study, the mucosal lesion close to a tooth treated with BAG+C, did not appear right after the application of the agent. Therefore, it is not clear whether it was caused by the tested agent or whether there was an injury, such as too intense tooth brushing, behind the event.
Conclusion
Our results indicated that the novel combination product BAG+C is, at least, as beneficial as BAG alone in treating residual pockets at the periodontal maintenance phase. In addition, the combination product decreased subjective sensitivity symptoms slightly more than the control product, thus, subjects may find BAG+C as a satisfactory treatment option. These findings are promising but understanding the limits of our investigation, further research is needed before suggesting its use in clinical practice.
Acknowledgement
This study was supported by Foundation for Finnish Inventions and by grant from Finnish Pharmaceutical Society. We wish to thank Helsinki University Faculty of Pharmacy, Division of Pharmaceutical Technology for productive discussions and help received. Especially, we thank Dr. Sari Airaksinen and Dr. Marikki Peltoniemi. We also wish to thank BonAlive Biomaterials Ltd, Finland and PharmaZell GmbH for providing the study material.
References
- Buencamino MC, Palomo L, Thacker HL (2009) How menopause affects oral health and what we can do about it. Cleve Clin J Med 76: 467-475.
- Isidor F, Karring T, Attström R (1984) Reproducibility of pocket depth and attachment level measurements when using a flexible splint. J Clin Periodontol 11: 662-668.
- Isidor F, Karring T, Attström R (1984) The effect of root planing as compared to that of surgical treatment. J Clin Periodontol 11: 669-681.
- Gillam DG, Tang JY, Mordan NJ, Newman HN (2002) The effects of a novel Bioglass dentifrice on dentine sensitivity: A scanning electron microscopy investigation. J Oral Rehabil 29: 305-313.
- Peltola M1, Aitasalo K, Suonpää J, Varpula M, Yli-Urpo A (2006) Bioactive glass S53P4 in frontal sinus obliteration: A long-term clinical experience. Head Neck 28: 834-841.
- Lindfors NC, Heikkilä JT, Koski I, Mattila K, Aho AJ (2009) Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater 90: 131-136.
- Lindfors NC, Hyvönen P, Nyyssönen M, Kirjavainen M, Kankare J, et al. (2010) Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 47: 212-218.
- Turunen T, Peltola J, Makkonen T, Helenius H, Yli-UrpO A (1998) Bioactive glass granules and polytetrafluoroethylene membrane in the repair of bone defects adjacent to titanium and bioactive glass implants. J Mater Sci Mater Med 9: 403-407.
- Tirapelli C, Panzeri H, Lara EH, Soares RG, Peitl O, et al. (2011) The effect of a novel crystallised bioactive glass-ceramic powder on dentine hypersensitivity: A long-term clinical study. J Oral Rehabil 38: 253-262.
- Stoor P, Söderling E, Grenman R (1999) Interactions between the bioactive glass S53P4 and the atrophic rhinitis-associated microorganism Klebsiella ozaenae. J Biomed Mater Res 48: 869-874.
- Hiltunen AK, Skogman ME, Rosenqvist K, Juvonen H, Ihalainen P, et al. (2016) Bioactive glass combined with bisphosphonates provides protection against biofilms formed by the periodontal pathogen Aggregatibacter actinomycetemcomitans. Int J Pharm 501:211-220.
- Montazeri AH1, Erskine JG, McQuaker IG (2007) Oral sodium clodronate induced osteonecrosis of the jaw in a patient with myeloma. Eur J Haematol 79: 69-71.
- Shoji K, Horiuchi H, Shinoda H (1995) Inhibitory effects of a bisphosphonate (risedronate) on experimental periodontitis in rats. J Periodontal Res 30: 277-284.
- Välimäki VV1, Moritz N, Yrjans JJ, Vuorio E, Aro HT (2006) Effect of zoledronic acid on incorporation of a bioceramic bone graft substitute. Bone 38: 432-443.
- Srisubut S, Teerakapong A, Vattraphodes T, Taweechaisupapong S (2007) Effect of local delivery of alendronate on bone formation in bioactive glass grafting in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104: e11-16.
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV (2007) Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000 43: 294-315.
- Tousi NS, Velten MF, Bishop TJ, Leong KK, Barkhordar NS, et al. (2013) Combinatorial effect of Si4+, Ca2+ and Mg2+ released from bioactive glasses on osteoblast osteocalcin expression and biomineralization. Mater Sci Eng C Mater Biol Appl 33: 2757-2765.
- Rosenqvist K, Airaksinen S, Fraser SJ, Gordon KC, Juppo AM (2013) Interaction of bioactive glass with clodronate. Int J Pharm 452: 102-107.
- Rosenqvist K, Airaksinen S, Vehkamäki M, Juppo AM (2014) Evaluating optimal combination of clodronate and bioactive glass for dental application. Int J Pharm 468: 112-120.
- Pandit N, Gupta R, Gupta S (2010) A comparative evaluation of biphasic calcium phosphate material and bioglass in the treatment of periodontal osseous defects: a clinical and radiological study. J Contemp Dent Pract 11: 25-32.
- Yadav VS, Narula SC, Sharma RK, Tewari S, Yadav RJ (2011) Clinical evaluation of guided tissue regeneration combined with autogenous bone or autogenous bone mixed with bioactive glass in intrabony defects. Oral Sci 53: 481-488.
- Sohrabi K, Saraiya V, Laage TA, Harris M, Blieden M, et al. (2012) An evaluation of bioactive glass in the treatment of periodontal defects: A meta-analysis of randomized controlled clinical trials. J Periodontol 83: 453-464.
- Mitsuta T1, Horiuchi H, Shinoda H (2002) Effects of topical administration of clodronate on alveolar bone resorption in rats with experimental periodontitis. J Periodontol 73: 479-486.
- Reddy MS, Weatherford TW 3rd, Smith CA, West BD, Jeffcoat MK, et al. (1995) Alendronate treatment of naturally-occurring periodontitis in beagle dogs. J Periodontol 66: 211-217.
- Yaffe A, Herman A, Bahar H, Binderman I (2003) Combined local application of tetracycline and bisphosphonate reduces alveolar bone resorption in rats. J Periodontol 74: 1038-1042.
- Igarashi K, Adachi H, Mitani H, Shinoda H (1996) Inhibitory effect of the topical administration of a bisphosphonate (risedronate) on root resorption incident to orthodontic tooth movement in rats. J Dent Res 75: 1644-1649.
- Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, et al. (2002) Bisphosphonates pamidronate and zolendronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 291: 680-686.
- Frith JC, Mönkkönen J, Auriola S, Mönkkönen H, Rogers MJ (2001) The molecular mechanism of action of the antiresorptive and anti-inflammatory drug clodronate: Evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 44: 2201-2210.
- Liu 1, Igarashi K, Kanzaki H, Chiba M, Shinoda H, et al. (2006) Clodronate inhibits PGE(2) production in compressed periodontal ligament cells. J Dent Res 85: 757-760.
- Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC (2007) Understanding the minimum clinically important difference: A review of concepts and methods. Spine J 7: 541-546.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 3000
- [From(publication date):
September-2017 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 2123
- PDF downloads : 877