Research Article Open Access
A Physician-Directed Commercial Low Calorie Diet with Intensive Behavioral Modification Decreases Metabolic Syndrome and Medication Use
| Bobbie G Paull-Forney1, Frank Dong2*, Justin B Moore1,2, Jeanene J Fogli3, Linda D Gotthelf3, James L Early2,3, Elizabeth Ablah2 | |
| 1Via Christi Weight Management, Via Christi Hospitals Wichita, Inc, USA | |
| 2University of Kansas School of Medicine-Wichita, Wichita, USA | |
| 3Health Management Resources, Boston, Massachusetts, USA | |
| Corresponding Author : | Frank Dong University of Kansas School of Medicine-Wichita 1010 N Kansas St, Wichita, KS, 67228, USA Tel: (316) 293-2627 Fax: (316) 293-2695 E-mail: fdong@kumc.edu |
| Received May 22, 2014; Accepted June 18, 2014; Published June 20, 2014 | |
| Citation: Paull-Forney BG, Dong F, Moore JB, Fogli JJ, Gotthelf LD, et al. (2014) A Physician-Directed Commercial Low Calorie Diet with Intensive Behavioral Modification Decreases Metabolic Syndrome and Medication Use. J Obes Weight Loss Ther 4:221. doi:10.4172/2165-7904.1000221 | |
| Copyright: © 2014 Paull-Forney BG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: In light of recent Centers for Medicare and Medicaid Services (CMS) recommendations on behavioral therapy for obesity, it is important to assess the outcomes of physician-directed low-calorie diet (LCD) interventions on metabolic risk factors. The aim of this study was to determine the effect of participation in a physiciandirected LCD program coupled with an intensive behavioral intervention on parameters of metabolic syndrome (MetS) and medication usage. Methods: This was a retrospective chart review of 445 participants, who completed at least 12 weeks in a community-based medical weight loss program. MetS was defined using the National Cholesterol Education Program/ Acute Treatment Panel III diagnostic criteria. Logistic regression analysis was conducted to identify factors predicting the MetS status at week 12. Results: Sixty eight percent of participants completing twelve weeks of the program achieved ≥10% initial body weight loss (IBWL). The mean weight loss is 11.8 %IBW. Proportions of participants meeting NCEP/ATP III criteria for MetS decreased for: waist circumference (100% at baseline vs 93.4% at week 12), triglycerides (51.8% at baseline vs 32.1% at week 12), fasting plasma glucose (60.1% at baseline vs 40.8% at week 12) and blood pressure (81.4% at baseline vs 61.1% at week 12). The prevalence of MetS decreased from 96% (n=248) at baseline to 67.8% (n=175) at week 12. Younger age, lower baseline BMI, and higher %IBWL were associated with an increased likelihood of MetS remission at week 12. Use of hypoglycemic and anti-hypertensive agents decreased by 27.5% and 12.8%, respectively, and doses of hypoglycemic and anti-hypertensive agents decreased for 75.3% and 40.4% of participants, respectively. Conclusions: A physician-directed low-calorie diet coupled with an intensive behavioral intervention is an effective option for achieving weight loss, improving individual metabolic risk factors, and reducing the overall need for hypoglycemic and anti-hypertensive medications.
| Keywords |
| Weight loss; Metabolic syndrome; Medication; Low calorie diet |
| Introduction |
| An estimated 35.7% of American adults are obese, defined as a body mass index (BMI) equal to or greater than 30 kg/m2 [1]. Obesity is strongly associated with many medical conditions, principally hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes mellitus, and especially the metabolic syndrome (MetS) [2,3]. The increase in the prevalence of obesity is the primary driver of the increasing prevalence of the MetS, defined as the elevation of at least three of the five measures: waist circumference (WC), triglyceride (TG) levels, fasting plasma glucose (FPG) levels, and blood pressure (BP), and/or decrease in high-density lipoprotein (HDL) levels. Each of these five measures is highly correlated with the others, and each is a significant risk factor for cardiovascular disease and type 2 diabetes mellitus. Direct and indirect medical expenditures in the United States (US) in 2008 for obesity-related illness were estimated to be at least $150 billion per year, exceeded only by costs associated with smoking [4,5]. |
| Because of the heavy burden of obesity on the US healthcare system, the U.S. Preventive Services Task Force has recommended that clinicians screen all adults for obesity and offer multicomponent behavioral interventions to affected individuals [6]. |
| In accordance with the USPSTF’s recommendations, the Centers for Medicare and Medicaid Services (CMS) recently approved physician reimbursement for provision of behavioral interventions through primary care offices consisting of 15 minute weekly counseling sessions for one month followed by every-other-week visits for another five months. Patients who lose ≥ 3 kg in the first six months (typically 14 sessions) are eligible for six additional monthly visits. |
| The precise methodology by which physician-directed behavioral intervention should occur remains uncertain. Lifestyle interventions, principally increasing physical activity and maintaining a low-fat diet are the recommended primary treatment of MetS [7,8]. Both shortand long-term studies of dietary interventions for obesity demonstrate that weight reduction reverses MetS, reduces individual cardiovascular risk factors, and decreases use of hypoglycemic and antihypertensive medication but these studies have largely been conducted in a controlled research environment [9-13]. To successfully counter the epidemic of obesity and its related morbidity, interventions must prove practical and effective in the community. The purpose of this study was to determine the effectiveness of a 12-week physician-directed commercial lowcalorie diet (LCD) and intensive behavioral intervention on patient weight and individual components and overall prevalence of the MetS, as well as associated medication use. |
| Methods |
| This was a retrospective analysis of a de-identified clinical dataset of adult patients (18 years of age and older) who voluntarily enrolled in and attended the first class of a physician-directed, community-based medical weight management program in a Midwestern city between January 1, 2009 and December 31, 2010. Health Management Resources® (HMR) is a comprehensive, high intensity lifestyle intervention that includes the use of meal replacements, increased physical activity, and weekly coaching. All participants met HMR Medical Guidelines which excludes pregnant women and individuals who are substance abusers, have eating disorders or behaviors, or who have been diagnosed with severe liver disease, renal failure, active malignancy, Cushing’s syndrome, bacterial endocarditis, osteomyelitis, or tuberculosis, and those who have undergone mal absorptive bariatric surgical procedures [14]. Individuals were also excluded from the analysis if their initial body mass index (BMI) was less than 25 kg/m2 or if they failed to complete 12 weeks of the intervention. The study was approved by the Institutional Review Board of the sponsoring medical center and by the Human Subjects Committee of the medical school. |
| Subjects were required to limit food choices to proprietary meal replacements (shakes, soup, cereal, and entrees) and to consume at least five meal replacements (shakes, soup, or cereal) along with two vitamin-mineral tablets per day. This regimen provided a minimum of 800 kilocalories per day as well as more than 100% of the recommended allowance of most vitamins and minerals [15]. Participants were required to attend weekly behavioral education classes and medical assessments and were encouraged to expend at least 300 kilocalories per day in planned physical activities. |
| Additionally, participants met individually with behavioral and medical staff prior to weekly group classes to report on calorie intake, exercise achievements, and to discuss barriers to program compliance or other concerns. Hypoglycemic, anti-hypertensive, and other medications were adjusted by the physician weekly, monthly, or less often as medically appropriate. Group classes were designed to provide support, accountability, and group problem solving for participants. Verified weights were recorded and entered into the clinical database every four weeks. Participant demographics and current medications were collected from detailed patient medical histories. |
| Study outcomes included the change in prevalence of individual MetS components (WC, TG, HDL, FPG, BP) and overall MetS diagnosis, and use of hypoglycemic and anti-hypertensive medications from baseline to immediately following the 12-week intervention. Fasting blood specimens were tested for FPG, TG, total cholesterol, HDL cholesterol, and calculated LDL cholesterol at baseline and immediately following 12 weeks of the intervention. Blood pressures were measured to the nearest 2 mmHg using an aneroid sphygmomanometer. Waist circumference was measured horizontally around the abdomen, immediately above the iliac crest to the nearest 0.1 inch, in accordance with the recommendations of NCEP/ATP III [7]. Heights were measured without shoes to the nearest 0.125 inch (0.32 cm). Verified body weights were measured to the nearest 0.1 pound (0.045 kg) with clothing and no shoes at baseline and at each treatment visit. Body mass index (BMI) was calculated as current mass in kilograms divided by the square of the current height in meters. Percent of Initial Body Weight lost (%IBWL) was calculated as: (baseline weight – week 12 weight) / baseline weight x 100. |
| Intake of meal replacements were self-reported to behavioral staff during weekly visits and verified by purchase of replacement meals. Participants were taught to calculate calories expended in physical activity based on intensity level, and values were self-reported to behavioral staff during weekly visits. Other variables collected included participant age, gender, race, and ethnicity. |
| Participants with three or more of the five MetS components were classified as having MetS [7]. Those who reported taking medication specifically to treat hypertension (β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, loop diuretics, or potassium-sparing diuretics) were classified as hypertensive and individuals who reported use of hypoglycemic agents (insulin or non-insulin agents) were classified as having elevated fasting glucose [16]. Similarly, participants reporting use of fibrates or nicotinic acid were classified as having elevated TG or low HDL [16]. |
| Statistical Analysis |
| All analyses were conducted using SAS Software for Windows (Version 9.3, Cary, NC). All data were analyzed on an aggregate level. Descriptive statistics were presented as means (M) and standard deviations (SD) for continuous variables, and frequencies and percentages for categorical variables. Paired student t-tests were used to assess selected baseline and week 12 parameters including weight, WC, FPG, TG, HDL, and systolic and diastolic BP. Logistic regression analysis was conducted to identify factors predicting the MetS remission status at week 12 (MetS vs non-MetS). These predictors include age, gender, ethnicity, baseline BMI category [overweight, class 1 obese, class 2 obese, or class 3 obese], and %IBWL. All statistical tests were two-sided. A p-value ≤ 0.05 was considered statistically significant. |
| Results |
| Of the 445 participants enrolled at baseline, 339 (76.2%) patients completed this 12-week program (hereafter referred as completer group) and 106 (23.8%) did not finish this 12-week program (hereafter referred to as the non-completer group). Of the non-completer group, 42 (40%) dropped within the first three weeks; mean length of stay was 5.8 weeks (SD 2.8). For the overall population, almost all patients were Caucasian and nearly two-thirds were women (Table 1). Neither the gender composition nor the race composition differ significantly between the completer group and the non-completer group (p=0.0723 for gender, and p=0.308 for race). More than half (67.9%) of patients were class 3 obese at baseline for the overall population, and the proportion of class 3 obese at baseline were 68.1% and 67% for these two groups respectively, though the difference is not statistically significant (p=0.9917). The average age was 49 ± 22 years for the overall population. There is a statistically significant difference of the age between these two groups (mean=50 years and 46 years respectively, p=0.0025). |
| The proportion of those with metabolic syndrome at baseline for the overall population was 79.7%. The proportion of those with metabolic syndrome for the completer group was statistically higher than that of the non-completer group (80.9% vs. 75.8%, p=0.0005). Additionally, the average percentages of initial body weight loss at 4 weeks and 8 weeks were compared between the completer and noncompleter group. For both time periods, the completer group achieved a statistically higher %IBWL than the non-completer group (p=0.0005 and p<0.0001 respectively). |
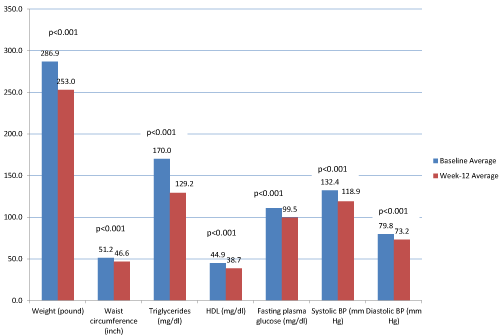
| For the completer group, 67.5% (n=229) achieved ≥ 10% IBWL at 12 weeks, consistent with the World Health Organization’s (WHO) and National Institutes of Health’s (NIH) definitions of successful weight loss [17,18]. The average weight loss after this 12-week program was 33.91 ± 14.75 pounds (15.39 ± 6.69 kilograms). Total weight, WC, TG, HDL, FPG, systolic BP and diastolic BP each significantly decreased from baseline to week 12 (p <0.001 for all seven lab/biometric outcome measures). With the exception of HDL, all six laboratory/ biometric outcome measures demonstrated clinical improvement (Figure 1). Proportions of participants meeting individual MetS criteria significantly changed from baseline to week 12: WC from 100% (n=106) to 93.4% (n=99), TG from 51.8% (n=162) to 32.1% (n=101), HDL from 64.4% (n=201) to 82.1% (n=256), FPG from 60.1% (n=190) to 40.8% (n=129), and BP from 81.4% (n=272) to 61.1% (n=204). The proportion of participants with a diagnosis of MetS changed from 96.1% (n=248) at baseline to 67.8% (n=175) at week 12 (Table 2). |
| Reported use of both hypoglycemic agents and anti-hypertensive medications decreased significantly from baseline to week 12 (p<0.001). The proportion of patients who take hypoglycemic agents decreased from 27% of participants (91 individuals) at baseline to 19.6% (66 individuals) at week 12. Use of insulin decreased from 8.6% (29 participants) at baseline to 5.9% (20 participants) at week 12. Among participants using insulin, 92.9% reduced their dose between baseline and week 12. Of all participants using hypoglycemic agents (insulin and non-insulin agents), 75.3% reported a reduction in dose between baseline and week 12. |
| Systolic blood pressure decreased by 13.96 ± 15.52 mmHg, and DBP by 7.55 ± 12.09 mmHg, and use of anti-hypertensive medications decreased from 58.9% (196 individuals) at baseline to 51.4% (171 individuals) at week 12. Of participants taking anti-hypertensive agents, 40.4% experienced a reduction in dose between baseline and week 12. At baseline, 9.8% (33 individuals) participants were using a fibrate and/ or niacin. This proportion did not significantly change by week 12. |
| To identify what type of patients would be most likely to achieve MetS remission at week 12, a preliminary logistic regression was conducted to identify possible predictors. These predictors included baseline obesity category, gender, race/ethnicity, % IBWL and age. Younger age, lower baseline BMI, and higher % IBWL were associated with an increased likelihood of MetS remission at week 12 (Table 3). A stepwise logistic regression analysis confirmed the effect of these identified factors. For every 5 years increase in age, the odds ratio of achieving MetS remission at week 12 decreased by 22% (95% CI=[0.69, 0.89]). For every 5% increase in the initial body weight loss percentage, the odds ratio of achieving MetS remission at week 12 increased by 61% (OR=1.61, 96% CI=[1.13, 2.31]). Compared with the class 3 obese patients, those who are overweight had the greatest odds of achieving MetS remission (OR=10.18, 95% CI=[1.66, 85.94]). The c (concordance) statistic for the logistic regression is 0.708, which represent an acceptable discrimination capacity. |
| Discussion |
| This study demonstrates that participants in a physician-directed community-based commercial 12-week weight loss program can achieve significant reductions in all parameters of MetS and medication usage. Additionally, we conclude that participants were most likely to achieve MetS remission at week 12 if they were younger, had a lower baseline BMI and had greater %IBWL. |
| Comparison of these results to published studies is problematic due to methodological differences, varying reporting methods, and inconsistent patient selection criteria and duration among studies. Nonetheless, evidence from previous studies is generally consistent with our results. A comprehensive meta-analysis reported average study duration of 32.1 ± 22.7 weeks and an average weight loss of 16.6 ± 12.6 kg [19]. This analysis concluded that during active weight loss, every one kilogram of body weight lost was associated with a total cholesterol decrease of 1.93 mg/dl (0.05 mmol/l), LDL decrease of 0.77 mg/dl (0.02 mmol/l), and TG decrease of 1.33 mg/dl (0.015 mmol/l) [19]. Triglyceride levels of participants in our study decreased two times more than predicted by the meta-analysis. This may be due to the varying nutritional make-up of the dietary interventions [20,21]. The acutely decreased HDL level in our study, though not optimal, is consistent with other studies of low-fat, hypocaloric diets. This study’s mean HDL decrease of 13.8% was 1.5 times more than projected by the meta-analysis, but their analysis suggested that for each kg of sustained weight loss, participants experienced a mean increase in the HDL level of 0.0347 mg/dl (0.009 mmol/l), arguing against a sustained downward effect on HDL [19]. |
| A meta-analysis of the effect of weight reduction on blood pressure suggested that, among participants with a mean age of 45-66 years and a mean weight reduction of 4.0 kg, a weighted mean difference of -4.5 mm Hg SBP and -3.2 mmHg DBP was observed, a per-kilogram reduction roughly equivalent to our results [22]. |
| The reduction in the proportion of participants with MetS in this study is consistent with published evidence that clinically significant weight reduction through a combination of dietary modification and physical activity can be effective in reducing the number of individuals meeting the criteria for MetS [23-25]. As individual components fall below diagnostic cut-points, the risk of cardiovascular disease and type 2 diabetes decreases [26,27]. |
| Our analysis suggests that younger, healthier individuals with lower baseline BMIs are more likely than their older, heavier, less healthy counterparts to achieve MetS remission via LCD and physical activity. This information has practical value; though earlier research has suggested that younger patients have somewhat higher attrition rates from weight management programs, physician-directed LCDs should perhaps be considered as an early treatment option for younger, healthier people with lower BMIs, prior to consideration of intensive pharmacologic or surgical management [28]. |
| This study has several limitations, including its retrospective design and its lack of a control group. Additionally, due to the participants in this study being in active weight loss, we cannot be confident that the improvements in FBG and TG resulted from weight loss alone; increased insulin sensitivity, decreased hepatic glucose production, and improved β cell function have been reported in as few as 10 days during a very low calorie diet when weight loss is still minimal [20,29,30]. The relatively short duration of follow-up limited the capacity to explore the sustainability of the LCD program. Inconsistent and limited evidence regarding conventional and LCD interventions suggests that approximately 20% of individuals who have lost weight sustain a loss of ≥ 10% of initial weight after one year [31,32]. |
| Traditionally, clinical trials of weight loss programs involve research participants who are compensated for their time and for completing the trial. While such studies are valuable in determining which approach results in the most significant weight loss, it cannot address the motivation to continue attending weekly classes and adhering to the prescribed diet in an ongoing clinical setting. The current study is unique in that it demonstrates that participants who are self-directed and motivated to pay for and attend a community-based weight loss program for at least 12 weeks experienced results that are similar, and in some cases superior, to those seen in a randomized control trial [22]. |
| Conclusions |
| A commercial low-calorie, low-fat diet coupled with an intensive behavioral intervention can be effective at reducing patient weight, improving individual risk factors, and reducing the overall need for hypoglycemic and anti-hypertensive medications. Physician-directed low-calorie, low-fat interventions, then, might represent an effective treatment approach for obesity and parameters of MetS regardless of whether reversal of MetS is achieved. MetS remission is more likely achieved among younger, healthier individuals with lower baseline BMIs. Further research is needed to determine predictors of sustainability and to reduce drop-out rates. |
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief : 1-8.
- Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res; 2000. 8: 605-19.
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, et al. (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837-1847.
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W (2009) Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 28: w822-831.
- Powers KA, Rehrig ST, Jones DB (2007) Financial impact of obesity and bariatric surgery. Med Clin North Am 91: 321-338, ix.
- Moyer VA, US. Preventive Services Task Force. (2012) Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157: 373-378.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation( 2002). 106:3143-3421.
- (1984) The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 251: 351-364.
- Flechtner-Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH (2010) Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev 26: 393-405.
- Furlow EA, Anderson JW (2009) A systematic review of targeted outcomes associated with a medically supervised commercial weight-loss program. J Am Diet Assoc 109: 1417-1421.
- Phelan S, Wadden TA, Berkowitz RI, Sarwer DB, Womble LG, et al. (2007) Impact of weight loss on the metabolic syndrome. Int J Obes (Lond) 31: 1442-1448.
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, et al. (2005) The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142: 611-619.
- Anderson JW, Conley SB, Nicholas AS (2007) One hundred pound weight losses with an intensive behavioral program: changes in risk factors in 118 patients with long-term follow-up. Am J ClinNutr 86: 301-307.
- Boston MA (2009) Health Management Resources Corporation. Health Management Resources. Guidelines for the Medical Management of Patients
- National Research Council, Recommended Dietary Allowances, 10th Edition.
- Grundy SM (2005) Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. ArteriosclerThrombVascBiol 25: 2243-2244.
- (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i-xii, 1-253.
- Clinical Guide lines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda (MD): National Heart, Lung, and Blood Institute 98-4083.
- Dattilo AM, Kris-Etherton PM (1992) Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J ClinNutr 56: 320-328.
- Malandrucco I, Pasqualetti P, Giordani I, Manfellotto D, De Marco F, et al. (2012) Very-low-calorie diet: a quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am J ClinNutr 95: 609-613.
- Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, et al. (2012) Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 307: 2627-2634.
- Siebenhofer A, Jeitler K, Berghold A, Waltering A, Hemkens LG, et al. (2011) Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database SystRev : CD008274.
- Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, et al. (2001) Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 134: 1-11.
- Anderssen SA, Carroll S, Urdal P, Holme I (2007) Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports 17: 687-695.
- Ilanne-Parikka P, Eriksson JG, Lindström J, Peltonen M, Aunola S, et al. (2008) Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care 31: 805-807.
- Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, et al. (2004) Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the CasaleMonferrato Study. Diabetes Care 27: 2689-2694.
- Sundström J, Vallhagen E, Risérus U, Byberg L, Zethelius B, et al. (2006) Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care 29: 1673-1674.
- Honas JJ, Early JL, Frederickson DD, O'Brien MS (2003) Predictors of attrition in a large clinic-based weight-loss program. Obes Res 11: 888-894.
- Henry RR, Scheaffer L, Olefsky JM (1985) Glycemic effects of intensive caloric restriction and isocaloricrefeeding in noninsulin-dependent diabetes mellitus. J ClinEndocrinolMetab 61: 917-925.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, et al. (1994) Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 17: 30-36.
- Wing RR, Phelan S (2005) Long-term weight loss maintenance. Am J ClinNutr 82: 222S-225S.
- McGuire MT, Wing RR, Hill JO (1999) The prevalence of weight loss maintenance among American adults. Int J ObesRelatMetabDisord 23: 1314-1319.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 13962
- [From(publication date):
June-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9405
- PDF downloads : 4557
