Short Communication Open Access
A One-Stop Novel Drug for Malaria Treatment and Control
Manoswini Dash and Aparup Das*Evolutionary Genomics and Bioinformatics Laboratory, National Institute of Malaria Research, Dwaraka, New Delhi, India
- *Corresponding Author:
- Aparup Das
Evolutionary Genomics and Bioinformatics Laboratory
Division of Genomics and Bioinformatics
National Institute of Malaria Research
Dwarka, New Delhi, India
Tel: +91 011 25307 322
Fax: +91 11 25307377
E-mail: aparup@mrcindia.org
Received date: February 11, 2016; Accepted date: February 22, 2016; Published date: February 27, 2016
Citation: Das A and Dash M (2016) A One-Stop Novel Drug for Malaria Treatment and Control. J Emerg Infect Dis 1:107. doi:10.4172/2472-4998.1000107
Copyright: © 2016 Dash M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Like many tropical diseases, malaria poses great threat to mankind in the under developed and developing countries. Control of malaria majorly depends on early diagnosis and thereafter effective treatment by antimalarials. However, malaria treatment is severely hampered by evolution and spread of drug-resistant malaria parasites, including the currently used drug artemisinin. Malaria control programs therefore need an antimalarial that can not only promptly kill the parasites with minimal side effect to humans, but also is affordable by the community and the chance of evolution of resistance is minimal. A wonder drug (named DDD107498) that can bear all the above properties has recently been developed and about to enter human clinical trial. Among other properties, (i) Effect on multiple species of malaria parasites at several life cycle stages (ii) Single dose effectiveness, (iii) Good pharmacokinetic properties and (iv) Low risk of parasite transmission due to active gametocyte clearance make the compound a perfect new-generation antimalarial.
Keywords
Malaria; Drug Studies; Chemo Protection; Transmission Blockage; Drug Resistance
Description
Malaria is a primordial disease that has been affecting human race since their origin. Albeit the parasite shows parallel divergence with hominids [1], it has evolved so finely and shaped its genome to a great extent to invade, dodge and damage hosts defence system. Small generation time, pressure to survive and grow under adverse environmental conditions inside host, ability to disguise and escape host immune system; help the parasite to succeed the evolutionary arms race [2]. Hence, malaria associated morbidity and mortality is a major public health concern especially for underdeveloped and developing countries of the globe. Though many malaria control and eradication strategies have been followed since ages, but none of them are successful in an overall control program. In the absence of a competent vaccine for malaria prevention and at the same time emerging resistance against currently available antimalarials, the ongoing malaria control programs have been severely hampered. Besides that, cross resistance among drugs due to their alike chemical combination is also well evidenced [3]. As a result of which, the current malaria control program has been adversely affected by the development and spread of parasite resistant strains to the working antimalarial, ACT (Artemisinin-based Combination Therapy) [4]. Therefore, the need of the hour is to develop a drug not only that has a quick and deep action in the parasite, but also by delaying the emergence of drug resistance. Bearing these facts in mind, Baragana and co-workers5 have recently designed a multiple stage antimalarial compound, which not only can treat malaria with single dose but also help in chemo-protection and blockage of transmission with less chance of development of resistance by the parasite.
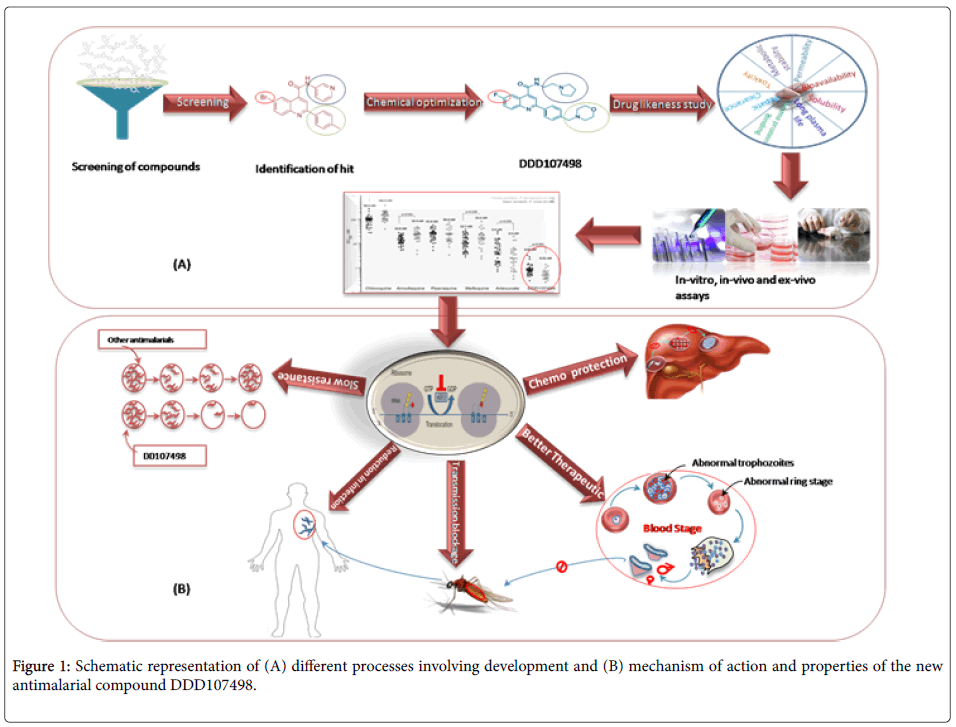
To initiate the process of development of such a compound, the scientific team [5] searched the chemical compound library at drug discovery unit (DDU) of University of Dundee that consists of around 4700 compounds and screened against blood stage asexual forms of the deadliest malaria parasite Plasmodium falciparum 3D7 strain. The screening process filtered out a compound class based on a 2,6- disubstituted quinoline-4-carboxamide scaffold, which seems to have activity against malaria parasite, although it shows poor physicochemical properties. The research team [5] further optimized the compound to attain drug-like properties through cycles of design, synthesis and testing. The significant optimization process included (i) Replacement of Bromine with Florine to reduce molecular mass and lipophilicity, (ii) Replacement of the 3-pyridyl substituent with an ethyl-pyrrolidine group and (iii) Addition of a morpholine group via methylene spacer. The final compound generated was named as DDD107498, and was experimented both in-vitro, in-vivo and ex-vivo methods using humanized mouse model and other model organisms against P.falciparum, P. vivax and P. berghei to confirm its efficacy. The compound was further evaluated for its drug-like properties, mode of action and safety measures. A comparative study with presently available drugs was also done to confirm its better efficacy, unique mechanism of action and ability to act at multiple stages of the parasite life cycle.
With a view to assess drug-like properties of DDD107498, several experiments were conducted, which provided evidences the compound had good metabolic stability, oral bioavailability, solubility, long plasma half-life, low protein binding and no or very less toxicity to the host system. The compound was further compared with presently available drugs (chloroquine, pyrimethamine, atovaquone and artemisinin) in terms of efficacy, dose concentration, and ability to reduce parasitaemia, and was found to be superior in each aspect from others. The minimum parasiticidal concentration of the compound was found to be 10-13 ng/ml. for asexual erythrocytic stage of infection using the blood of P. falciparum 3D7 infected NOD-scid IL-2R_null mice engrafted with human erythrocytes (Figure 1).
Further, stage-specificity study of P. falciparum using synchronized cultured in SCID mouse model revealed the fatal activity of the compound in each stage of the malaria parasite. At the concentration of 4 nM for 24 hours, the compound ceased production of normal trophozoite from ring stage, prevented schizont formation from trophozoites, and ring formation from schizont with reduction of parasite, providing evidence of its efficacy to hinder growth and development of all asexual blood stages of the parasite. Furthermore, the compound interrupted the life cycle of parasite at multiple stages (pre-erythrocytic, erythrocytic and post erythrocytic), meaning greater chemo-protection and transmission blockage characteristics. Specifically, it was found that the molecule can act on the intrahepatocyte parasites, thereby could be considered as a chemoprotective agent as well as gametocytes-disrupting, blocking malaria transmission process. To this respect, the compound was found to be more efficient than currently used atovaquone along with proguanil as a chemo-protective agent and also provides protection during intermittent infection. The chemo-protective potential of the compound was assessed in vivo by treating the mouse with DDD107498 at 3 mg/kg. Two hour later, the mouse was infected with P. berghei and there was no sight of parasitemia after 30 days. This demonstrates that the compound is a potent chemo-protective agent. In addition, the team performed standard membrane feeding assay and used P. berghei infected mouse model to unravel its role and strong potential to block transmission.
Since the important aspect of drug development lies in the evaluation of toxicity of the drug to humans, various experiments were performed to evaluate toxicity of the newly developed compound DDD107498. The concentration at which the compound reduces maximum parasitemia was exposed to human cell lines (hepatocytes and MRC5) and it was found that the compound was non-toxic to human cell even at higher concentration. Also, it neither caused excess inhibition nor induction to the activity of Cytochrome P450 isoforms, therefore, probability of drug-drug interaction was fairly negligible. This is because, Cytochrome P450 and its isoforms serve important role in drug metabolism process and its excess inhibition or induction hampers the drug efficacy. In addition, very low inhibitory response to other ion channels, non-mutagenic characters and long half-life of DDD107498 have made it fairly promising single-dose cure that can provide once-in-a-week chemo-protection against malaria parasite infection.
With the knowledge on the strong potentiality of the DDD107498 compound that can be an effective antimalarial, the research team5 further searched for the target of action of this compound in the malaria parasite P. falciparum. This is important, as the efficacy of the compound at each stage of parasite development could be fully acknowledged once the actual target site in the parasite is known. For this, the team5 first developed P. falciparum resistant strains by continuously exposing DDD107498 to the drug sensitive (3D7) and multi drug resistant (7G8 and Dd2) P. falciparum strains. Thereafter, whole genome sequencing of both the sensitive and resistant strains followed by comparative genomic analyses pointed towards a gene named PfeEF2 (Plasmodium falciparum translation elongation factor 2) which had mutations in all parasite strains resistant to DDD107498 but not in the susceptible ones. The function of the protein expressed by the gene PfeEF2 was identified to play a major role in the protein synthesis process by translocating the ribosome along the mRNA in P. falciparum. As a further confirmation measure, a metabolic labelling assay was performed which revealed that the strains which developed resistance to DDD107498 showed quite normal protein synthesis while in wild types protein synthesis was greatly reduced (Figure 2).
In addition, fluorescent microscopy assay of transgenic parasites expressing wild type and mutant PfeEF2 revealed endogenous expression of PfeEF2, which further confirmed the gene PfeEF2 as the primary molecular target of DDD107498. These chains of experiments therefore provided evidence that the DDD107498 compound inhibits protein synthesis in cytoplasm which is contrary to drugs like tetracycline and azithromycin whose site of action is the apicoplast of the parasite.
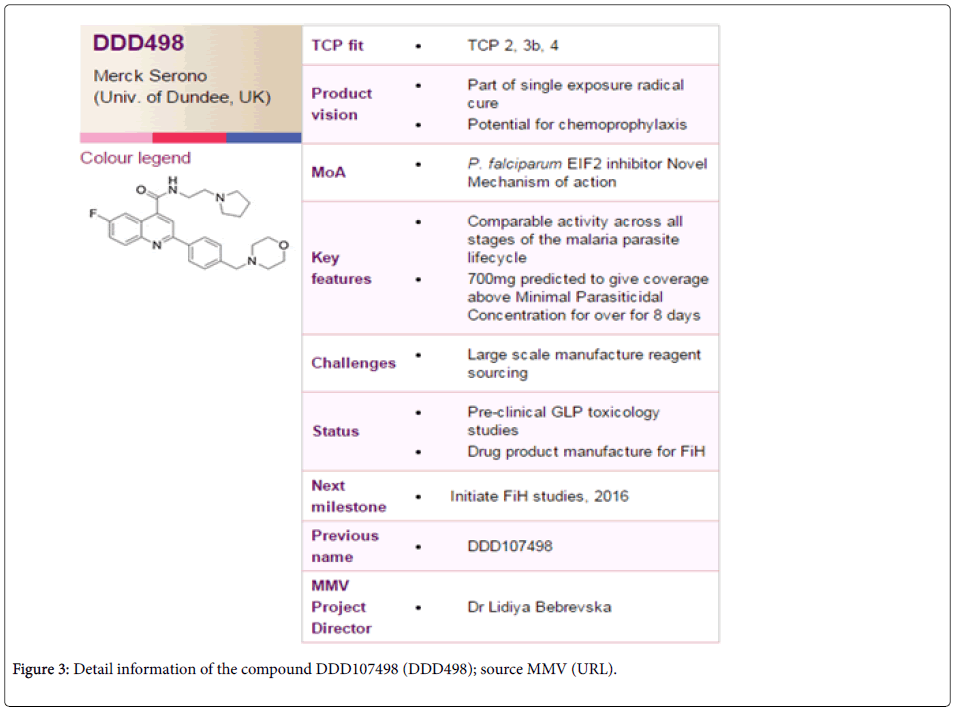
How important is the discovery of such a new-generation antimalarial compound to the control program? Worldwide malaria control measures principally rely on prompt effective treatment, as this not only reduces morbidity due to malaria, but the available parasites in the population for transmission is also minimized [6]. However, malaria treatment is majorly hampered by non-availability of a suitable antimalarial that can stay in the control program for long [7] due to quick evolution and spread of resistant forms of the malaria parasites [8]. Although several antimalarials have been used at different time points which had proved highly efficient in reducing malaria mortality and morbidity to a great extent, evolution and spread of parasites resistant to almost all the antimalarials had grossly hampered malaria control programs of the endemic countries of the globe [9]. In this concern, the antimalarial compound DDD107498 appears to be promising with its ability to work (i) On different genus of plasmodium (P. falciparum, P. vivax, P. berghei and P. yoelli ), (ii) On different stages of parasite life cycle, (iii) At very minimum concentration (iv) With very less or no toxicity in human and (v) Having different mode of action than the presently used antimalarials make it a promising antimalarial of the future. Furthermore, (vi) Possession of good pharmacokinetics property and (vii) Global monomorphic nature of the target gene PfeEf2 make this antimalarial not only with no/minimal side effect to human, but also with less chance of immediate evolution of resistance by the malaria parasites (Figure 3).
The principal property of the DDD107498, which is now undergoing safety testing through medicines for malaria venture (MMV) with a view to entering human clinical trials within the next year, lies in its potential to treat malaria with a single dose and very cost effective (~1 USD for a dose), preventing malaria transmission to a significant extent in populations. As with every other newly developed antimalarial compound, it remains to be seen that how DDD107498 behaves in the field once it enters into human clinical trials. Also, like other antimalarials, this molecule must follow combination therapy with a fast acting compound to control initial infection [10]. Therefore, immense care should be taken while choosing the second compound, as efficacy of the DDD107498 might also be greatly influenced by the partner drug. Whatever the case may be, on the basis of promises so far DDD107498 has shown [5], once cleared by all ethical guidelines; it is expected to prove to be a boon for malaria treatment and control in future.
Acknowledgement
The authors thank the Director of the National Institute of Malaria Research (NIMR), for facilities and the Indian Council of Medical Research (ICMR), New Delhi for a Senior Research Fellowship to M. Dash.
References
- Carter R, Mendis KN (2002) Evolutionary and historical aspects of the burden of malaria. Clinical Microbiology Reviews 15: 564-594.
- Sadd BM, Schmid-Hempel P (2009) A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biology Letters 5:798-801.
- Bollenbach T (2015) Antimicrobial interactions: mechanisms and implications for drug discovery and resistance evolution. Current Opinion in Microbiology 27: 1-9.
- Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, et al. (2015) Spread of artemisinin-resistantPlasmodium falciparumin Myanmar: a cross-sectional survey of the K13 molecular marker. THE LANCET Infectious Diseases 15: 415-421.
- Baragan˜a B, Hallyburton I, Lee MCS, Norcross NR, Grimaldi R, et al. (2015) A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522:315-320.
- Phillips RS (2001) Current status of malaria and potential for contro.ClinMicrobiol Rev 14: 208-226.
- Vial H, Taramelli D, Boulton IC, Ward SA, Doerig C, et al. (2013) CRIMALDDI: platform technologies and novel anti-malarial drug targets. Malaria Journal 12: 396.
- Das A, Dash A (2007) Evolutionary paradigm of chloroquine-resistant malaria in India. Trends Parasitol23: 132-135.
- Awasthi G, Das A (2013) Genetics of chloroquine-resistant malaria: a haplotypic view. Memorias Do InstitutoOswaldo Cruz108: 947-961.
- White NJ (2004) Antimalarial drug resistance. J Clin Invest 113: 1084-1092.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11509
- [From(publication date):
June-2016 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 10512
- PDF downloads : 997