A Novel SCN9A Gene Mutation in a Patient with Carbamazepine-Resistant Paroxysmal Extreme Pain Disorder
Received: 18-Nov-2015 / Accepted Date: 08-Dec-2015 / Published Date: 16-Dec-2015 DOI: 10.4172/2572-4983.1000S1004
Abstract
Paroxysmal extreme pain disorder is an autosomal dominant disorder caused by mutation of the SCN9A gene. In most cases, the pain is relieved by carbamazepine. We report on a novel SCN9A mutation associated with carbamazepine-resistant. The proband was a 7-month-old child who suffered from typical attacks from birth onwards. Sequencing of SCN9A revealed a heterozygous c.4880T>G substitution. Identification of this novel mutation and characterization of the associated carbamazepine-resistant phenotype may facilitate diagnosis and drug development.
Keywords: Nerve pain; Mutation; Paroxysmal extreme pain disorder
Abbreviations
PEPD: Paroxysmal Extreme Pain Disorder; SCN9A: Sodium Channel, Voltage-gated, Type IX, Alpha Subunit
Introduction
Paroxysmal extreme pain disorder is a very rare disease concerning only a few families in the world. Affected patients experience excruciating pain (centered on the rectal, ocular and/or mandibular regions) that is generally provoked by defecation, bowel movements, cold wind, yawning and eating. Pain is associated with autonomic manifestations as skin flushing and harlequin color changes. Tonic attacks and syncope with apnea and bradycardia are described in infancy and young children and might be confused with epilepsy. Between episodes, patients frequently suffer of constipation, because of fear of inducing a pain attack by the passage of stool. The onset of attacks of paroxysmal extreme pain disorder is in the neonatal period or in early infancy, but with increasing age, the rectal attacks become generally less frequent although ocular and jaw attacks may become more troublesome. Paroxysmal extreme pain disorder is caused by mutation of the sodium channel, voltage-gated, type IX, alpha subunit (SCN9A) gene encoding the NaV1.7 channel [1].
Different gain-of-function mutations in SCN9A are responsible for a number of autosomal dominant neuropathic pain syndromes such as small fiber sensory neuropathy and primary erythromelalgia. Paroxysmal extreme pain disorder is a rare disorder of pain and autonomic dysfunction that presents with four painful episodes: birth crisis, rectal crisis, ocular crisis and mandibular crisis. Onset is usually in the neonatal period or infancy. Primary erythromelalgia is characterized by childhood or adolescent onset of episodic symmetrical red congestion, vasodilatation, and burning pain of feet and legs provoked by exercise and exposure to warmth. In these disorders, mutations correspond to a single amino-acid substitution, whereas homozygous loss-of-function mutations as truncating or non-sense mutations are associated with congenital insensitivity to pain. In contrast to the other pain syndromes linked to SCN9A, most patients with paroxysmal extreme pain disorder are relieved by carbamazepine, an antiepileptic, sodium-channel-blocking drug.
Case Study
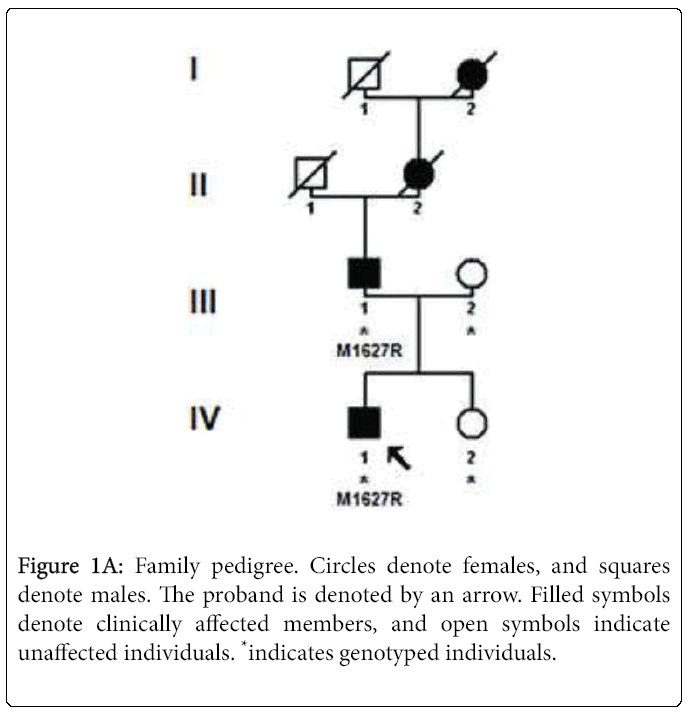
In February 2008, a father and son presented with a familial history of episodes of excruciating rectal pain. The proband was a 7-monthold child who had experienced attacks of paroxysmal extreme pain with sudden stiffening, startling, inconsolable screaming, harlequin color changes affecting the left lower limb, and (in some instances) loss of contact and upward deviation of the eyeballs from birth onwards. The attacks were variously triggered by defecation, wiping the perineal area or inserting a thermometer into the anus. Periauricular attacks (triggered by cold food ingestion) and ocular attacks (triggered by eye rubbing) began at the age of one year.
Carbamazepine was introduced at the age of 5 months. The dose was progressively increased to 35 mg/kg/d but was only modestly effective. The drug was withdrawn after 11 months due to the onset of tonic non-epileptic seizures, which were considered to be adverse drug reaction. Gabapentin was ineffective. Carbamazepine was then reintroduced at the age of 13 months but did not relieve the pain. A dose increase to 50 mg/kg/d was associated with sedation, prompting the parents to withdraw the drug after 8 months of treatment. The parents estimated that carbamazepine had merely halved the frequency of the rectal attacks (from one a day to one every two days).
Carbamazepine had no effect on facial attacks. Topiramate was ineffective and was associated with sleepiness and aggressiveness; the drug was rapidly discontinued. Only high-dose of oxcarbazepine (30 mg/kg/d) was partially effective on the rectal attacks; it suppressed the attacks triggered by defecation but not those triggered by a fall on the buttocks. Moreover, oxcarbazepine had no effect on the ocular attacks. Between attacks, the proband suffered from severe constipation due to reluctance to pass stools for fear of inducing a painful attack. Although constipation was treated with osmotic laxatives and nutritional monitoring, chronic fecal leakage and two episodes of fecal impaction occurred. Around the age of one year, an essential tremor was observed as seen in the proband's father and paternal grandmother. The proband is now 8 years old and is still taking oxcarbazepine (30 mg/kg/d). Facial attacks still occur daily but have decreased in intensity. Rectal attacks have also decreased in frequency (to about one a year) with treatment, and because the proband has learnt to pass stools slowly without any pushing effort. Despite constant treatment with laxatives, he still suffers from severe constipation and fecal leakage and has not yet acquired cleanliness.
The proband’s father (aged 41) described sudden, rapidly progressive episodes of excruciating rectal pain with a trigger area below the navel, including the genitalia, perineal region, and upper thighs. He had never been diagnosed so far but felt that he and his son must be suffering from the same condition. He reported similar experiences by his mother and his maternal grandmother. However, these family members have not been examined in our department and lack a molecular diagnosis. Rectal and facial attacks had started very early in his first year of life. Rectal attacks were triggered by sudden blows, cuts, scratches, and pinches. Mild attacks lasted 10 seconds and involved only one limb, whereas the most severe attacks lasted 20 seconds and tended to affect both limbs. The pain was described as an internal ripping sensation first centered on the anal region and then spreading to the perineum, buttocks, genitalia, and then one or both legs. The father reported feeling limp and unresponsive in the minutes immediately following an attack. Attacks were followed by a refractory period of around 30 minutes. He initially suffered up to two attacks a day, although there was a marked fall in frequency and severity with age. He also described attacks triggered by yawning. These affected the mandibular and periauricular regions, and were accompanied by hypersalivation. With age, facial attacks became less frequent too. At the time of first consultation, he had been free of any attacks for several years. He never had taken any medication for this condition.
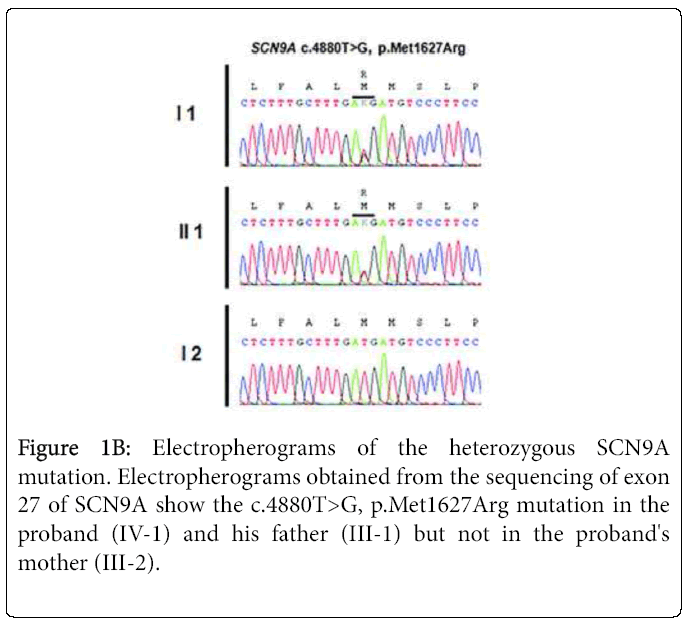
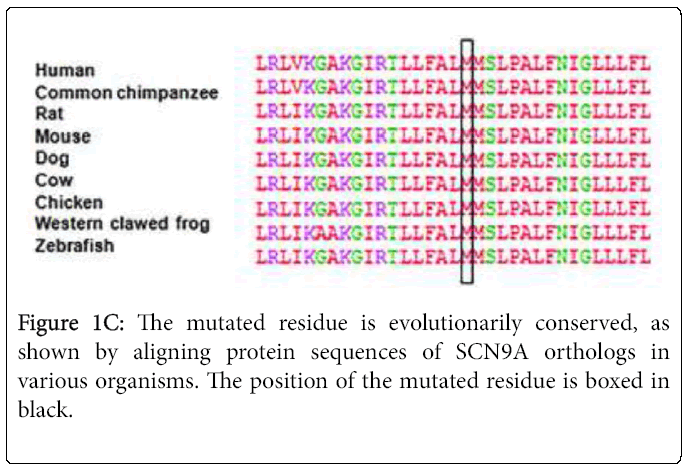
Sequencing of SCN9A's coding exons revealed a heterozygous c. 4880T>G substitution in exon 27 in both patients but not in the unaffected mother and daughter (Figures 1A and 1B). This would result in replacement by arginine of the conserved methionine 1627 residue located within the linker joining segments S4 and S5 in domain IV of the Nav1.7 channel (Figure 1C). The c.4880T>G substitution was not found in 200 healthy controls or in the dbSNP, 1000 Genomes Project or Exome Variant Server databases. The SIFT, PolyPhen-2, MutationTaster and Align GVGD prediction tools all suggested that Met1627Arg was likely to affect protein function. Lastly, another mutation of the same amino acid (Met1627Lys) has been reported in carbamazepine-responsive paroxysmal extreme pain disorder [2,3].
Discussion
Primary erythromelalgia and paroxysmal extreme pain disorder among painful inherited neuropathies are caused by several mutations in the SCN9A gene [4,5]. Met1627Lys mutation has been previously described in a sporadic case of paroxysmal extreme pain disorder from France [1], and then reported in an English family [2]. In both families, carbamazepine was effective with low-doses in two adult women (200 to 600 mg/d) reducing the frequency of attacks to just one a year. The M1627R mutation described here is very similar to M1627K with substitution of methionine 1627 by a conservative residue. Such a difference in carbamazepine response between M1627K and M1627R cases is very surprisingly. The (adult) patients with the M1627K mutation were carbamazepine-responsive with 600 mg/d, whereas a dose of 400 mg/d in a two-year-old boy weighing 8 kg merely halved the frequency of the pain attacks to one every two days. A number of voltage-clamp studies have shown that the M1627K mutation has a marked effect on fast inactivation of NaV1.7 [1-3] and resurgent sodium currents [3]. However none of these electrophysiological studies assessed the effect of carbamazepine in the M1627K mutant. The essential role played by the Nav1.7 channel in pain makes it a drug target for the treatment of neurological pain [6,7]. The design of new selective sodium channel antagonists that stabilize inactivated states of the channel may represent a promising strategy [8]. Identification of this novel SCN9A mutation and characterization of the associated carbamazepine-resistant phenotype may facilitate diagnosis and drug development.
Declaration of Interest
The authors report no conflicts of interest
References
- Fertleman CR, Ferrie CD, Aicardi J, Bednarek NA, Eeg-Olofsson O, et al. (2007) Paroxysmal extreme pain disorder (previously familial rectal pain syndrome). Neurology 69: 586-595.
- Dib-Hajj SD, Estacion M, Jarecki BW, Tyrrell L, Fischer TZ, et al. (2008) Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol Pain 4: 37.
- Theile JW, Jarecki BW, Piekarz AD, Cummins TR (2011) Nav1.7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navbeta4 peptide-mediated resurgent sodium currents. The Journal of Physiology 589: 597-608.
- Cummins TR, Dib-Hajj SD, Waxman SG (2004) Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci 24: 8232-8236.
- YangS, Xiao Y, Kang D, Liu J, Li (2013) Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proceedings of the National Academy of Sciences of the United States of America 110: 17534-17539.
- Yang Y, Dib-Hajj SD, Zhang J, Zhang Y, Tyrrell L, et al. (2012) Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel. Nat Commun 3: 1186.
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, et al. (2015) Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 350: aac5464.
Citation: Sablonnière B, Huin V, Cuvellier J, Genet A, Dhaenens C, et al. (2015) A Novel SCN9A Gene Mutation in a Patient with Carbamazepine-Resistant Paroxysmal Extreme Pain Disorder. Neonat Pediatr Med 1: S1004. DOI: 10.4172/2572-4983.1000S1004
Copyright: © 2015 Sablonniere B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3659
- [From(publication date): 0-2015 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2880
- PDF downloads: 779