Research Article Open Access
A Novel Methodology for the Quantification of B-Vitamers in Breast Milk
Karine Meisser Redeuil*, Sylvie B√?¬©net, Michael Affolter, Sagar Thakkar K and Esther Campos-Gim√?¬©nezNestl√?¬© Research Center, Nestec Ltd., Lausanne, Switzerland
- *Corresponding Author:
- Karine Meisser Redeuil
Nestl√?¬© Research Center
Verschez-les-Blanc, PO Box 44
1000 Lausanne 26, Switzerland
Tel: +41217859229
Fax: +41217859486
E-mail: Karine.Meisser@rdls.nestle.com
Received date: February 24, 2017; Accepted date: February 27, 2017; Published date: March 02,2017
Citation: Redeuil KM, B√?¬©net S, Affolter M, Thakkar SK, Campos-Gim√?¬©nez E (2017) A Novel Methodology for the Quantification of B-Vitamers in Breast Milk. J Anal Bioanal Tech 8:352. doi: 10.4172/2155-9872.1000352
Copyright: © 2017 Redeuil KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
With this report we present development, validation and application of an analytical methodology for the quantification of 18 water soluble vitamers and secreted or biological forms in breast milk. On a relatively low amount of breast milk (200 µL), we applied isotope dilution-based sample preparation based on a combination of enzymatic treatment and protein precipitation using acidic methanol enriched with stable isotope labelled internal standards. Compounds separation was achieved by reversed-phase liquid chromatography and detection performed by tandem mass spectrometry in positive electrospray ionization mode. To perform the quantification of 18 water soluble vitamers, procured pooled breast milk was used to build matrixmatched calibration curves, as labelled internal standards were not available for each vitamer. The analytical approach has been validated according to the EMA guidelines. The overall performance of the method was considered adequate, with 0.3- 28.3% and 0.9-32.6% intra and inter-day precision respectively and averaged accuracy reaching 92.2-107.5%. In addition, performed freeze/thaw stability studies showed the potential degradation of some vitamers. We therefore recommend particular attention in sample collection with rather having dedicated aliquots with small volumes. The feasibility of this analytical approach has been evaluated by quantifying various breast milk samples that were procured from an external supplier. The main forms found in breast milk were thiamine monophosphate for B1, flavin adenine nucleotide for B2, nicotinamide for B3, pyridoxal for B6 and 5-methyl tetrahydrofolic acid for B9. In addition, we newly reported nudifloramide as B3 form present in breast milk. With this analytical approach, it will give more confidence to provide a comprehensive assessment of the presence of water soluble vitamins in breast milk. This will enable the accurate evaluation of the nutritional requirements of infants.
Keywords
Water soluble vitamins; Stability; Breast milk; Liquid chromatography; Tandem mass spectrometry
Abbreviations: 5-Me THF: 5-methyl Tetrahydrofolic acid; Ac. Ac.: Acetic Acid; CAN: Acetonitrile; Asc. Ac.: Ascorbic Acid; BM: Breast Milk; CE: Collision Energy; DTT: Dithiothreitol; EtOH: Ethanol; FA: Folic Acid; FAD: Flavin Adenine Dinucleotide; FMN: Flavin Mononucleotide; Frag: Fragmentor; F/T: Freeze/thaw; HFBA: Heptafluorobutyric Acid; HPLC: High Performance Liquid Chromatography; ISTD: Internal Standard; LC-MS: Liquid Chromatography-Mass Spectrometry; LCMS/ MS: Liquid Chromatography-Tandem Mass Spectrometry; LLOD: Lower Limit of Detection; LLOQ: Lower Limit of Quantification; MeOH: Methanol; MP: Mobile Phase; NA: Nicotinic Acid; NM: Nicotinamide; NUA: Nicotinuric Acid; Nudi: Nudifloramide; p-ABGA: para- Aminobenzoyl Glutamic Acid; PFP: Pentafluorophenyl; PL: Pyridoxal; PLP: Pyridoxal 5’-Phosphate; PM: Pyridoxamine; PMP: Pyridoxamine 5’-Phosphate; PN: Pyridoxine; Pyr: Pyridoxic 4-acid; Qual: Qualifier; Quant: Quantifier; QC: Quality Control; Ribo: Riboflavin; RT: Retention Time; S/N: Signal/Noise Ratio; STD: Standard; Th: Thiamine; TMP: Thiamine Monophosphate; UV: Ultraviolet.
Introduction
B-vitamins are essential for the physical growth and cellular metabolism of infants [1-7]. Their adequate level plays a key role in avoiding deficiencies and health risks of humans at all stages of life. Prominent example includes but not limited to are, ariboflavinosis [8], pellagra [9], cardiovascular risk [10], cognitive impairment [11] and neural tube defect [12,13].
WHO recommends exclusive breastfeeding to new-born infants up to 6 months of age followed introduction of complementary food in addition to continued breastfeeding until two years or even beyond [1]. In exclusively breastfed infants, breast milk (BM) is the sole source of these essential vitamins required at various steps of growth and development. Presumably, the breast milk provides most the indispensable nutrients. However, even with supplementation, BM vitamin D amounts may not be enough and so the recommendation of vitamin D supplementation in infants in at least the first year of life is favoured [14]. In order to understand the infant’s vitamin intake, it is essential to study the evolution of these vitamins in BM. Composition of vitamin B1 in BM has been shown to changes over a lactation period of 40 days [15,16] however there is only limited or conflicting knowledge regarding the composition of most water soluble vitamins in breast milk along the lactation [17]. Acquiring knowledge on the vitamin composition of breast milk is of vital importance to our understanding of the nutritional needs of new-born infants rapidly growing. Research in the field of maternal and infant nutrition can only be possible with accurate and reliable analytical methodologies to precisely identify and quantify all vitamers (various forms of a vitamin) present in breast milk.
To this date several studies investigating B-vitamins in breast milk have been reported in the literature. However individual/separate methods have been used to identify/quantify each B-vitamin and/or related vitamer and thus these procedures required a relatively large volume (approximately 25 mL) of BM [18]. Microbiological or liquid chromatography-ultraviolet (UV)/fluorescence methods have been traditionally used for the characterization of BM vitamers [19,20] or human plasma [21] to quantify a single vitamin. They present the disadvantage of providing a result on a total vitamin, without the possibility to differentiate between the native vitamin used in food supplementation and its secreted (or biological) form present in BM. Multi-analyte methods emerged in the past few years with the advantages of reducing time for sample preparation and more importantly reduced sample volume. The technique of choice is liquid chromatography hyphenated with tandem mass spectrometry (LC-MS/ MS) which demonstrated reliable results in quantification of multivitamin in various matrices, such as food [22-24], supplements [25,26], human serum or plasma [27] and BM [28,29].
For instance, in 2012, Hampel et al. quantified four different water soluble vitamins (B1, B2, B3, B6) represented by six compounds in breast milk by LC-MS/MS [28]. More recently, Ren et al. validated a LCMS/ MS based method to quantify six different B-vitamins (B1, B2, B3, B5, B6 and B8)) by analysing ten vitamers [29]. It is indeed of crucial importance to identify and accurately quantify each secreted vitamer in BM. For example, vitamin B1 main form secreted in breast milk is thiamine monophosphate (TMP), contributing for about 60% of total vitamin B1 [30] and which is involved in Krebs cycle [2,3], whereas thiamine is the compound used for fortification/supplementation.
We decided to focus our work on the quantification of five major B-vitamins: B1, B2, B3, B6 and B9 represented by 18 compounds. We therefore designed an analytical methodology to simultaneously quantify these 18 B-vitamers using relatively low volume of human breast milk (200 μL). To the best of our knowledge, this is the first time these many B-vitamers are quantified in BM from a relatively low volume of milk that requires a single sample preparation and a single analytical run. We are confident that this methodology will find application in large cohort studies to investigate the role of these vitamers in health and disease.
Materials and Methods
Chemicals and reagents
HPLC grade water, LC-MS grade acetonitrile (ACN), LC grade methanol (MeOH), LC grade ethanol (EtOH), LC grade isopropanol, and dithiothreitol (DTT) were obtained from Merck (Darmstadt, Germany). Acetic acid (Ac. Ac.) for analysis, ascorbic acid (Asc. Ac.) and LC grade heptafluorobutyric acid (HFBA), α-amylase from Bacillus subtilis were from Fluka/Sigma-Aldrich Chemie GmbH (Buchs, Switzerland). Normal Wistar rat serum was purchased at LucernaChem (Zug, Switzerland).
Standards and stable isotope labelled standards were purchased from various suppliers: Thiamine monophosphate (TMP) chloride dihydrate, flavin mononucleotide (FMN) sodium salt hydrate, flavin adenine dinucleotide (FAD) disodium salt, nicotinamide (NM), nicotinic acid (NA), nicotinuric acid (NUA), pyridoxal 5’-phosphate (PLP) hydrate, pyridoxal (PL) hydrochloride, pyridoxamine 5’-phosphate (PMP), pyridoxic 4-acid (Pyr) from Sigma-Aldrich Chemie GmbH. Pyridoxamine (PM) dihydrochloride, para-aminobenzoyl glutamic acid (p-ABGA) from Fluka/Sigma-Aldrich Chemie GmbH. Thiamine hydrochloride, riboflavin, pyridoxine (PN) hydrochloride from Supelco (Buchs, Switzerland), nudifloramide (N-methyl-2-pyridone- 5-carboxamide) from Toronto Research Chemicals (Toronto, Canada), folic acid (FA), 5-methyl tetrahydrofolic acid (5-Me THF) and 5-methyl tetrahydrofolic diglutamic acid from Schircks Laboratories (Jona, Switzerland).
Nicotinamide-[d4], nicotinic acid-[d4], nicotinuric acid- [d4], pyridoxine-[d2] from CDN isotopes (Darmstadt, Germany). Pyridoxamine-[d3], riboflavin-[13C4,15N2], thiamine-[13C4] hydrochloride from Isosciences (King of Prussia, USA). Nudifloramide- [d3], para-aminobenzoyl glutamic acid-[d4] (p-ABGA-[d4]) from Toronto Research Chemicals. Folic acid-[13C5] and 5-methyl tetrahydrofolic acid-[13C5] (5-Me THF-[13C5]) from Merck Eprova (Schaffhausen, Switzerland).
Milk samples for method validation and application
A pooled breast milk, 10 single donor breast milk as well as pooled breast milk samples (colostrum and >4 weeks of lactation) was procured from LeeBio Solutions (St. Louis, MO, USA). Pooled breast milk was aliquoted and stored at –80°C prior to analysis. In addition, 2 milk-based breast milk substitutes with known vitamin content were analysed on each validation day.
Standard solutions
Based on solubility/stability studies, individual standards and internal standard (ISTD) for method validation were weighed and dissolved in different solvents or solutions as reported previously [31]. Individual standard stock solutions were then combined and diluted with H2O to obtain the standard solution mixes to build up the calibration curve. Individual ISTD stock solutions were combined and diluted with H2O to obtain an ISTD mix. ISTD mix was prepared in order to spike the matrix to obtain a final ISTD concentration corresponding to STD6, equivalent to 50 × STD1 analyte concentration.
Sample preparation
Breast milk samples (pooled and single donor samples) were thawed at room temperature for 1 h and vortexed for homogenization. An aliquot of 200 μL was taken into an amber vial and spiked with 200 μL of an α-amylase solution (0.25 mg/mL). Samples were left at room temperature for 30 min. Then, 20 μL of ISTD mix (or 20 μL of STDs mix containing all ISTDs in order to build the calibration curve and QC samples in the matrix) were added and followed by addition of 600 μL of methanol containing 1% Ac. Ac. The mixture was vortexed for 10 s and left to stand for 5 min at room temperature to allow protein precipitation. The samples were centrifuged at 21,000 × g for 10 min at 4°C in a Sigma 3-16K centrifuge. The supernatant was transferred into amber vials and evaporated under a stream of gaseous nitrogen flow at room temperature. Dried extracts were reconstituted in 500 μL of mobile phase A based solution (H2O containing 5% Ac. Ac. 0.2% HFBA and 1% Asc. Ac. (v/v)). The samples were vortexed for 10 min and filtered through an Ultrafree Centrifugal filter, PVDF 0.45 μm at 12000 g for 3 min at room temperature. Filtered extracts were finally transferred into amber vial prior to injection.
LC-MS/MS Analysis
Chromatographic separation of water soluble vitamers was achieved on a reversed-phase column ACE-3 C18-pentafluorophenyl (C18-PFP), 150 × 2.1 mm, 3 μm at room temperature using an Agilent 1290 Infinity system at a constant flow rate of 0.2 mL/min. Mobile phase A was water containing 5% acetic acid and 0.2% HFBA (v/v), mobile phase B was acetonitrile. Final mobile phase gradient is described elsewhere [31]. Finally, 10 μL of sample extract were injected into the LC-MS/ MS system. Mass spectrometry was performed on an Agilent 6460 MS mass spectrometer using Jetstream mode applying positive ionization.
Method validation
All method validation procedure has been followed according to EMA Guidelines.
Compound selectivity: Retention time (RT) of each analyte was first evaluated by injecting individual standard solutions. Defined quantifier and qualifier transitions for each analyte were used to calculate qualifier/ quantifier ratio, which is analyte dependent. Detailed parameters are summarized in Table 1. This ratio was specified in the quantification method with an accepted tolerance according to EU Commission Decision 2002/657/EC in order to eliminate false positives. Non-spiked sample with and without ISTD were also prepared to follow possible interferences.
Calibration curve and quality controls: Matrix-matched calibration was applied for each analyte with its dedicated internal standard to enable the quantification of quality control (QC) and single donor samples.
The curves of each analyte comprised a concentration range of nearly three orders of magnitude starting from endogenous concentrations. A standard mix with adjusted concentrations depending on the endogenous content of each analyte present in the BM pool was prepared and used to build all calibration points according to the following levels: standard 1 (STD1), 2.0 × STD1, 5.0 × STD1, 12.5 × STD1, 25 × STD1, 50 × STD1, 100 × STD1, 175 × STD1 and 250 × STD1. STD1 level was selected to be about 20% of the endogenous content, which was the limiting factor and falling in the range of instrumental lower limit of quantification (LLOQ). Each of these solutions was used to spike the BM pool dedicated to the method validation, as described in section 3.4. Calibration curves were constructed after subtraction of peak area ratio of endogenous content from peak area ratios of points at increasing concentrations. Outliers were marked and excluded from calibration process when individual point deviated by more than 20% (25% in case of lowest calibration point) from theoretical value. No more than 1/3 of calibration points could be excluded per analyte for each analytical series. QC samples were prepared in the same way according to the following enrichment levels: 6 × STD1, 40 × STD1, 150 × STD1.
Recovery and ion suppression: A standard mix with adjusted concentrations depending on the endogenous content of each analyte present in the BM pool was prepared and used to build all preextraction QCs according to the following levels: QC mid=40 × STD1, QC high=150 × STD1.
To investigate the efficacy of the sample preparation, the analyte recovery and ion suppression values were determined by comparing the peak areas between three types of samples as previously described [31]. Recovery studies were carried out by individually preparing and quantifying QC sample 4 times (n=4) in a row within one batch at each level and the average of all results was reported. Note that the calculation of ion suppression was performed after deduction of the endogenous analyte content measured after the calibration process by quantifying a non-spiked sample (matrix without analyte spike).
Accuracy and precision: To evaluate the trueness of the method, accuracies of measured concentrations in QC samples and 2 breast milk substitute were calculated. Measured concentrations of QC at low, mid and high levels were calculated applying the calibration curve after deduction of the endogenous analyte content. Theoretical and experimentally measured concentrations from these QC samples were compared for each analyte. They were evaluated in duplicate at each level on each day of validation (n=6). Measured concentrations of each of the vitamins in the infant formula powders were compared to known values.
To evaluate the performance of the method, intra-day and interday precision of measured concentrations in quality control samples were expressed as coefficient of variation (%). Due to the presence of endogenous amounts for most of the compounds, we decided to select three appropriate QC enrichment levels distributed over the calibration curve (namely 6 × STD1, 40 × STD1 and 150 × STD1). Intra-day and inter-day repeatability studies were carried out by individually preparing and quantifying each QC level in duplicate on six consecutive days (n=6). Results were calculated using an in-house software applying classical statistics.
Freeze-Thaw (FT) stability of vitamers BM samples: The effect of freeze/thaw cycles on water soluble vitamin related compounds in BM samples was evaluated in various conditions: cycle 0 (FT 0), pooled BM was freshly spiked at QC levels mid and high and directly prepared and analysed on the same day; cycle 1 (FT 1), BM samples were spiked at QC levels mid and high and frozen at –80°C for at least 24 h and then thawed on the preparation day; cycle 2 (FT 2), BM samples were spiked at QC levels mid and high and frozen at –80°C for at least 24 h and thawed at room temperature and kept for 4 h on the bench before they were put back to the freezer. They were finally thawed on the preparation day; cycle 3 (FT 3), cycle 4 (FT 4) and cycle 5 (FT 5) with the same procedure. An analytical series (calibration points, QC samples and internal reference samples) was freshly prepared and analysed, including the preparation of QC samples coming from the various freeze/thaw cycles. Stability of the compounds was evaluated based on calculated accuracy toward the theoretical spiked value. Compounds were considered stable if accuracy percentage was falling into the range 80-120%.
Presence of polyglutamate forms of folate in BM
Due to controversy on the presence of folate polyglutamates in BM [32], additional experiments have been designed to evaluate if polyglutamate forms of folates are present in BM. Rat serum (containing the enzyme with glutamic acid cleavage activity or deconjugase) incubation time was optimized based on previous data in milk powder (data not shown) to obtain the highest release effect. BM samples were then prepared following the optimized sample preparation protocol with or without deconjugase.
Results and Discussion
LC-MS parameter optimization
The optimization on the chromatography has been reported elsewhere [31]. The mass spectrometric behaviour of all standards and internal standards was studied using positive-ion ESI. Optimization of the mass spectrometric conditions (fragmentor (frag) voltage and collision energy (CE)) was carried out by infusing the analyte solutions individually and automatically. Chromatography dependent mass spectrometric parameters such as ion source and curtain gases pressure, gas temperature were optimized stepwise, manually, by infusing standard mix solutions targeting specific compounds of major interest at specific percentage of mobile phase B, which is summarized in Table 1.
| Analyte | Molecularweightoffreeform [Da] |
Q1[m/z] | QuantQ3 [m/z] |
QualQ3 [m/z] |
RetentionTime [min] |
Frag.V | QuantCE | QualCE | Qual/Quantratio | InstrumentalLLODs [pg] |
InstrumentalLLOQs [pg] |
InstrumentalLLODs [pg/mL] |
InstrumentalLLOQs [pg/mL] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiamine | 265.35 | 265.1 | 122.0 | 42.0 | 8.9 | 78.0 | 9.0 | 50.0 | 19.1 | 0.200 | 0.600 | 20.0 | 60.0 |
| TMP | 345.33 | 345.3 | 121.9 | 42.0 | 5.9 | 85.0 | 16.0 | 50.0 | 19.2 | 0.400 | 1.200 | 40.0 | 120.0 |
| Riboflavin | 376.36 | 377.1 | 243.1 | 172.1 | 6.8 | 132.0 | 21.0 | 41.0 | 36.6 | 0.400 | 1.200 | 40.0 | 120.0 |
| FAD | 785.55 | 786.1 | 348.0 | 136.0 | 5.0 | 165.0 | 12.0 | 40.0 | 73.2 | 0.900 | 2.700 | 90.0 | 27.0 |
| FMN | 456.34 | 457.1 | 439.0 | 359.0 | 5.5 | 140.0 | 12.0 | 20.0 | 33.6 | 0.400 | 1.200 | 40.0 | 120.0 |
| Nicotinamide | 122.12 | 123.0 | 80.0 | 78.0 | 4.7 | 94.0 | 21.0 | 25.0 | 36.5 | 0.375 | 1.125 | 37.5 | 112.5 |
| Nicotinicacid | 123.11 | 124.0 | 80.0 | 78.0 | 4.4 | 94.0 | 21.0 | 25.0 | 97.7 | 0.150 | 0.450 | 15.0 | 45.0 |
| Nicotinuricacid | 180.16 | 181.2 | 134.9 | 79.0 | 5.2 | 100.0 | 16.0 | 48.0 | 41.2 | 0.080 | 0.240 | 8.0 | 24.0 |
| Nudifloramide | 152.15 | 153.2 | 107.9 | 109.9 | 3.1 | 100.0 | 20.0 | 20.0 | 57.6 | <0.625 | <1.875 | <62.5 | <187.5 |
| Pyridoxine | 169.18 | 170.1 | 152.1 | 134.0 | 6.3 | 78.0 | 9.0 | 17.0 | 103.6 | 0.004 | 0.012 | 4.0 | 12.0 |
| Pyridoxamine | 168.19 | 169.1 | 152.1 | 134.1 | 8.2 | 76.0 | 9.0 | 17.0 | 79.1 | 0.020 | 0.060 | 20.0 | 60.0 |
| Pyridoxal | 167.16 | 168.1 | 150.1 | 94.0 | 5.9 | 56.0 | 5.0 | 20.0 | 25.0 | 0.400 | 1.200 | 40.0 | 120.0 |
| PLP | 247.14 | 248.1 | 150.0 | 94.0 | 2.6 | 122.0 | 12.0 | 28.0 | 30.2 | 0.063 | 0.188 | 6.3 | 18.8 |
| PMP | 248.17 | 249.2 | 134.0 | 231.9 | 3.9 | 85.0 | 20.0 | 8.0 | 74.3 | 0.075 | 0.225 | 7.5 | 22.5 |
| 4-Pyridoxicacid | 183.16 | 184.2 | 165.9 | 147.9 | 3.5 | 85.0 | 8.0 | 20.0 | 111.5 | 0.100 | 0.300 | 10.0 | 30.0 |
| FolicAcid | 441.40 | 442.2 | 295.1 | 176.0 | 7.6 | 90.0 | 10.0 | 40.0 | 25.8 | 0.150 | 0.450 | 15.0 | 45.0 |
| 5-MeTHF | 459.55 | 460.2 | 313.1 | 180.0 | 7.5 | 108.0 | 14.0 | 42.0 | 23.2 | 0.100 | 0.300 | 10.0 | 30.0 |
| p-ABGA | 266.25 | 267.3 | 119.9 | 91.9 | 5.4 | 76.0 | 16.0 | 44.0 | 27.2 | 0.030 | 0.090 | 3.0 | 9.0 |
| Thiamine-[13C4] | 269.32 | 269.1 | 122.1 | -- | 8.9 | 90.0 | 10.0 | -- | -- | -- | -- | -- | -- |
| Riboflavin-[13C4,15N2] | 382.33 | 383.1 | 249.1 | -- | 6.8 | 127.0 | 20.0 | -- | -- | -- | -- | -- | -- |
| Nicotinamide-[d4] | 126.12 | 127.1 | 84.0 | -- | 4.7 | 94.0 | 24.0 | -- | -- | -- | -- | -- | -- |
| Nicotinicacid-[d4] | 127.11 | 128.1 | 84.0 | -- | 4.4 | 94.0 | 24.0 | -- | -- | -- | -- | -- | -- |
| Nicotinuricacid-[d4] | 184.16 | 185.2 | 139.0 | -- | 5.2 | 94.0 | 16.0 | -- | -- | -- | -- | -- | -- |
| Pyridoxine-[d2] | 171.18 | 172.2 | 154.0 | -- | 6.3 | 84.0 | 12.0 | -- | -- | -- | -- | -- | -- |
| Pyridoxamine-[d3] | 171.22 | 172.1 | 155.1 | -- | 8.2 | 76.0 | 8.0 | -- | -- | -- | -- | -- | -- |
| Folicacid-[13C]5 | 446.40 | 447.1 | 295.0 | -- | 7.6 | 92.0 | 10.0 | -- | -- | -- | -- | -- | -- |
| 5-MeTHF-[13C]5 | 464.46 | 465.2 | 313.1 | -- | 7.5 | 120.0 | 15.0 | -- | -- | -- | -- | -- | -- |
| p-ABGA-[d4] | 270.25 | 271.3 | 123.9 | -- | 5.4 | 76.0 | 16.0 | -- | -- | -- | -- | -- | -- |
Table 1: MS parameters for the detection of water soluble vitamers.
Extraction optimization
The objective of sample preparation was to maximize recovery of compounds of interest in one single preparation as well as reducing ion suppression effects [32-34]. The biggest challenge was to recover all compounds at once, given the diversity of physicochemical properties, which is presented in Figure 1. Optimisation was focused on recovering compounds known to be the main forms present in BM. Protein precipitation is a common practice to pre-treat BM samples [35] and methanol has become the organic solvent of choice to precipitate proteins as reported in previous findings [29,36]. In addition, our previous findings on other matrices [31] guided us to select methanol as organic solvent of choice. Addition of water and α-amylase prior to protein precipitation and acetic acid with methanol helped getting a higher recovery for most compounds (data not shown). Ultimately, acidified methanol (1% acetic acid) was selected as the best extraction mixture. Ultrafiltration cleared sample extracts to avoid column blocking. The reconstitution solvent volume was 500 μL and its composition was defined as MP A based solution (H2O containing 5% Ac. Ac, 0.2 HFBA and 1% Asc. Ac (v/v)).
Method validation
Compound selectivity: Compound selectivity was studied by combining retention time data with qualifier (qual)/quantifier (quant) ratio for each analyte, for each injection. Each analytical series also contained non-spiked samples to confirm the absence of potential interferences. This allowed the exclusion of false positives.
Matrix-matched calibration: By applying the specific criteria of signal/ noise (S/N)=3.3 for the lower limit of detection (LLOD) and S/N=10 for the LLOQ, instrumental LLOD and LLOQ for each analyte was evaluated after injecting standard solutions as described in section 3.3. All results are summarized in Table 1.
Due to the lack of labelled internal standards for each of the 18 compounds present in the methodology, at least one labelled internal standard was attributed to its B-vitamin subclass. The detailed internal standard attribution is specified in Table 2. Quantification was performed as described elsewhere [31].
| Analyte | Fittedcalibration | AppliedWeighting | Selectedinternalstandard | Endogenouscontentinthematrix | Calibratedrangeinbreastmilk[ng/mL] | Averagedcalibration[r2] | Recovery[%] | IonSuppression[%] |
|---|---|---|---|---|---|---|---|---|
| Thiamine | linear | 1/x | Thiamine-[13C4] | yes | 5-1250 | 0.9995 | 92.8% | -13.0% |
| TMP | linear | 1/x | Thiamine-[13C4] | yes | 10-2500 | 0.9940 | 90.2% | -47.4% |
| Riboflavin | linear | 1/x | Riboflavin-[13C4,15N2] | yes | 10-2500 | 0.9989 | 84.1% | -26.4% |
| FAD | linear | 1/x | Riboflavin-[13C,15N] 42 |
yes | 30-7500 | 0.9771 | 82.1% | -25.9% |
| FMN | linear | 1/x | Riboflavin-[13C4,15N2] | yes | 2-500 | 0.9951 | 84.9% | -31.9% |
| Nicotinamide | linear | 1/x | Nicotinamide-[d4] | yes | 30-7500 | 0.9986 | 88.1% | -68.8% |
| Nicotinicacid | linear | 1/x | Nicotinicacid-[d4] | no | 2-500 | 0.9995 | 88.2% | -42.4% |
| Nicotinuricacid | linear | 1/x | Nicotinuricacid-[d4] | no | 0.2-50 | 0.9993 | 89.6% | -27.2% |
| Nudifloramide | linear | 1/x | Nicotinamide-[d4] | yes | 50-12500 | 0.9954 | 89.8% | -82.3% |
| Pyridoxine | linear | 1/x | Pyridoxine-[d2] | no | 0.2-50 | 0.9988 | 94.3% | -16.6% |
| Pyridoxamine | linear | 1/x | Pyridoxamine-[d3] | yes | 0.5-125 | 0.9993 | 104.2% | -22.6% |
| Pyridoxal | linear | 1/x | Pyridoxine-[d2] | yes | 10-2500 | 0.9941 | 90.4% | -49.2% |
| PLP | linear | 1/x | Pyridoxine-[d2] | yes | 5-1250 | 0.9945 | 71.2% | -51.4% |
| PMP | linear | 1/x | Pyridoxamine-[d3] | yes | 0.5-125 | 0.9931 | 116.4% | -55.6% |
| 4-Pyridoxicacid | linear | 1/x | Pyridoxine-[d2] | yes | 2-500 | 0.9943 | 81.2% | -90.9% |
| FolicAcid | linear | 1/x | Folicacid[13C5] | yes | 2-500 | 0.9992 | 82.7% | -43.3% |
| 5-MeTHF | linear | 1/x | 5-MeTHF[13C5] | yes | 0.5-125 | 0.9989 | 87.8% | -51.9% |
| p-ABGA | linear | 1/x | p-ABGA-[d4] | no | 0.2-50 | 0.9983 | 91.5% | -34.1% |
Table 2: Validation parameters for the quantification of water soluble vitamers in human milk.
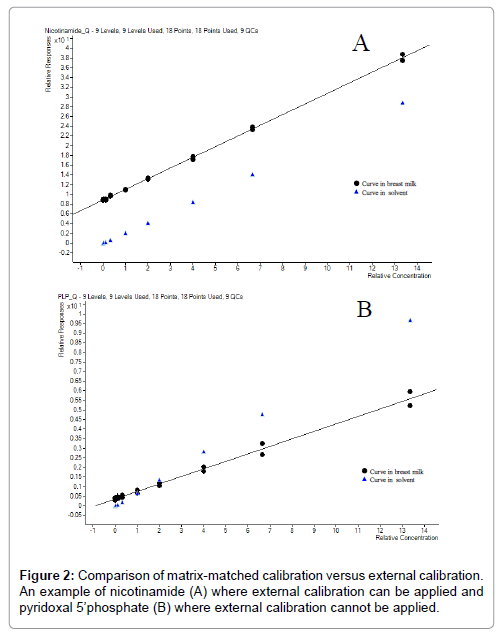
Matrix-matched calibration and external calibration were strictly evaluated. Comparison was made between quantification applying external calibration curve and matrix-matched calibration curve to decide whether matrix-matched calibration was necessary as more tedious than external calibration. A typical calibration curve of nicotinamide is shown in Figure 2A and pyridoxal 5’-phosphate in Figure 2B. These results show that nicotinamide could have been quantified with external curve (as curve in solvent and in matrix are parallel and the difference correspond the nicotinamide endogenous content). It is explained by the fact that nicotinamide having its labelled internal standard would not require matrix-matched calibration as reported before [37]. However, PLP (not possessing any labelled internal standard) curves clearly indicate that the presence of matrix effects are varying depending on the concentration level. The external calibration could not be applied in this case. Due to the complexity of the matrix, the high number of compounds to investigate and the absence of labelled internal standard for each of the compounds of interest, the quantification is best performed applying matrix-matched calibration curves for all compounds. In a previous report a BM like matrix system was used [36]. We opted for the use of pooled BM to build the calibration curves and QC samples in our analytical approach. Fitting coefficients and calibrated ranges for individual compounds are detailed in Table 2.
Recovery, ion suppression, accuracy and precision: The analyte recovery and ion suppression were calculated as described in the experimental section. Recovery and ion suppression results are summarized in Table 2 and those of accuracy and precision in Table 3. Only PLP exhibited low recovery compared to others with an average at 71.2%, which was the compromise we had to make to cope with compound diversity. Ion suppression was reduced after having adjusted reconstitution volume to 500 μL (instead of 200 μL initially set).
| Analyte | Intra-dayprecisionat6*STD1level[%] (n=6) |
Intra-dayprecisionat40*STD1level[%] (n=8) |
Intra-dayprecisionat150*STD1level[%] (n=8) |
Inter-dayprecisionatendogenouslevel[%] (n=6) |
Inter-dayprecisionat6*STD1level[%] (n=6) |
Inter-dayprecisionat40*STD1level[%] (n=7) |
Inter-dayprecisionat150*STD1level[%] (n=7) |
Averagedaccuracy [%] |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiamine | 1.2 | 0.3 | 3.8 | 3.9 | 3.1 | 2.5 | 6.7 | 101.7 | ||||||
| TMP | 14.8 | 4.3 | 3.8 | 13.1 | 16.9 | 7.6 | 6.7 | 98.7 | ||||||
| Riboflavin | 2.8 | 2.8 | 1.9 | 2.7 | 3.0 | 4.0 | 2.5 | 102.9 | ||||||
| FAD | 16.0 | 6.0 | 1.4 | 6.8 | 18.5 | 8.5 | 5.1 | 95.2 | ||||||
| FMN | 14.9 | 4.4 | 1.9 | 12.7 | 12.7 | 4.1 | 3.2 | 95.1 | ||||||
| Nicotinamide | 3.1 | 1.5 | 1.4 | 1.8 | 3.5 | 2.3 | 2.5 | 98.5 | ||||||
| Nicotinicacid | 0.6 | 1.1 | 10.1 | 3.0 | 0.9 | 1.6 | 10.2 | 97.9 | ||||||
| Nicotinuricacid | 7.9 | 2.1 | 2.4 | 30.5 | 10.7 | 2.9 | 2.5 | 101.3 | ||||||
| Nudifloramide | 9.5 | 7.9 | 2.2 | 5.5 | 15.1 | 8.7 | 3.9 | 100.3 | ||||||
| Pyridoxine | 7.3 | 1.0 | 0.7 | 2.2 | 5.5 | 1.3 | 1.0 | 93.8 | ||||||
| Pyridoxamine | 3.2 | 0.5 | 0.9 | 3.8 | 3.3 | 1.1 | 0.9 | 99.2 | ||||||
| Pyridoxal | 7.9 | 1.5 | 2.5 | 2.9 | 9.9 | 5.7 | 3.5 | 107.5 | ||||||
| PLP | 5.7 | 3.4 | 7.7 | 12.2 | 13.7 | 9.6 | 9.2 | 92.2 | ||||||
| PMP | 8.3 | 8.2 | 10.3 | 15.3 | 10.9 | 8.1 | 11.7 | 92.9 | ||||||
| 4-Pyridoxicacid | 28.3 | 24.4 | 9.7 | 15.4 | 32.6 | 25.5 | 8.1 | 102.8 | ||||||
| FolicAcid | 3.0 | 2.3 | 1.6 | 6.5 | 4.3 | 3.1 | 2.2 | 105.8 | ||||||
| 5-MeTHF | 5.4 | 2.2 | 2.9 | 4.9 | 6.2 | 2.1 | 2.7 | 99.0 | ||||||
| p-ABGA | 9.1 | 3.1 | 3.2 | 61.0 | 9.0 | 4.0 | 4.0 | 99.3 | ||||||
| Average | 8.3 | 4.3 | 3.8 | 11.3 | 10.0 | 5.7 | 4.8 | 99.1 | ||||||
| Analyte | Intra-dayprecisiononpowderedmilkA [%](n=6) |
Inter-dayprecisiononpowderedmilkA [%](n=6) |
Intra-dayprecisiononpowderedmilkB [%](n=6) |
Inter-dayprecisiononpowderedmilkB [%](n=6) |
AveragedaccuracyonpowderedmilkA [%] |
AveragedaccuracyonpowderedmilkB [%] |
||||||||
| Thiamine | 0.9 | 1.6 | 0.5 | 1.8 | 98.3 | 115.7 | ||||||||
| TMP | -- | -- | -- | -- | -- | -- | ||||||||
| Riboflavin | 1.5 | 1.9 | 1.2 | 1.3 | 112.1 | 96.0 | ||||||||
| FAD | -- | -- | -- | -- | -- | -- | ||||||||
| FMN | -- | -- | -- | -- | -- | -- | ||||||||
| Nicotinamide | 1.2 | 1.3 | 0.7 | 1.1 | 93.7 | 99.8 | ||||||||
| Nicotinicacid | -- | -- | -- | -- | 96.3 | |||||||||
| Nicotinuricacid | -- | -- | -- | -- | ||||||||||
| Nudifloramide | -- | -- | -- | -- | ||||||||||
| Pyridoxine | 1.2 | 1.8 | 0.8 | 1.5 | 104.3 | |||||||||
| Pyridoxamine | -- | -- | -- | -- | -- | -- | ||||||||
| Pyridoxal | -- | -- | -- | -- | -- | -- | ||||||||
| PLP | -- | -- | -- | -- | -- | -- | ||||||||
| PMP | -- | -- | -- | -- | -- | -- | ||||||||
| 4-Pyridoxicacid | -- | -- | -- | -- | -- | -- | ||||||||
| FolicAcid | 2.4 | 2.6 | 2.2 | 2.5 | 106.1 | 128.9 | ||||||||
| 5-MeTHF | -- | -- | -- | -- | -- | -- | ||||||||
| p-ABGA | -- | -- | -- | -- | -- | -- | ||||||||
| Average | 1.4 | 1.9 | 1.1 | 1.7 | 101.3 | 108.9 | ||||||||
Table 3: Performance parameters for the quantification of water soluble vitamers.
Effect of several freeze/thaw (F/T) cycles on vitamer stability in BM samples
Samples from a cohort study or a clinical study that takes a year or two for enrolment of the study subjects are rarely analysed immediately upon collection. Sampling, post collection sample treatment and freeze/ thaw cycles may have an impact on analytical results. Averaged accuracy were calculated using 2 QC levels (mid and high level) at various F/T cycles based on theoretical spiked value. Results on the effect of thawing, refreezing and rethawing BM samples are presented in Table 4. Majority of the compounds evaluated are not affected by freeze/thaw cycles. However, PLP (B6 vitamer) and 5-Me THF (B9 vitamer) are affected by these cycles. 5-Me THF level already decreases at the first thaw cycle whereas PLP started being unstable after 4 cycles. The presence of a glutamic unit on 5-Me THF may suggest that the observed degradation comes from a peptidase activity cleaving the amino acid unit. Results also suggest that folic acid starts degrading but additional experiments should confirm this trend. FMN (B2 vitamer) and PMP (B6 vitamer) concentration are increasing with the freeze/thaw cycles. It is known that FAD can degrade into FMN [33], which is probably the phenomenon occurring here. This is however not visible in the FAD concentration due to the fact that concentration range of FAD is 15 times higher than FMN concentration range. PMP concentration (qualifier ratio confirms the identity of the compound) increasing with the number of freeze/ thaw cycles may be due to other compounds present in the matrix that are affected by the time. In addition, the presence of phospholipase and phosphatase in BM may lead to the degradation of these compounds. These results demonstrate how key it is to have reliable procedures on sample collection and storage in clinical trials. Based on these findings, it is recommended to avoid F/T cycles as much as possible during sample analysis to any clinical trial dealing with B-vitamin analysis in BM. We therefore recommend that these conditions should clearly be addressed during protocol building and that the identical procedure is followed for all subjects over the duration of the clinical trial. Indeed to this end, it would be ideal if smaller aliquots of appropriate quantity of BM required per analytical method be built immediately after sample collection before any freezing.
| Analyte | AveragedaccuracyatFT0[%] | AveragedaccuracyatFT1[%] | AveragedaccuracyatFT2[%] | AveragedaccuracyatFT3[%] | AveragedaccuracyatFT4[%] | AveragedaccuracyatFT5[%] |
|---|---|---|---|---|---|---|
| Thiamine | 99.3 | 103.3 | 104.0 | 113.2 | 104.4 | 115.8 |
| TMP | 97.8 | 99.1 | 82.2 | 102.0 | 90.2 | 69.3 |
| Riboflavin | 104.0 | 104.2 | 106.2 | 117.1 | 114.0 | 125.4 |
| FAD | 98.9 | 91.9 | 85.8 | 105.2 | 61.9 | 125.0 |
| FMN | 99.6 | 122.5 | 124.6 | 163.1 | 138.6 | 174.0 |
| Nicotinamide | 99.1 | 98.1 | 99.1 | 103.8 | 99.3 | 116.4 |
| Nicotinicacid | 99.4 | 98.8 | 101.2 | 109.6 | 100.3 | 111.6 |
| Nicotinuricacid | 98.3 | 98.2 | 102.7 | 102.0 | 98.3 | 106.0 |
| Nudifloramide | 97.5 | 97.7 | 98.3 | 102.1 | 101.4 | 99.8 |
| Pyridoxine | 98.9 | 98.6 | 101.7 | 100.8 | 97.4 | 112.8 |
| Pyridoxamine | 99.5 | 89.3 | 104.5 | 104.5 | 115.7 | 122.6 |
| Pyridoxal | 103.4 | 106.4 | 106.9 | 105.1 | 103.3 | 123.0 |
| PLP | 102.2 | 97.4 | 99.9 | 82.4 | 53.4 | 64.8 |
| PMP | 95.5 | 104.2 | 124.9 | 163.9 | 167.9 | 231.2 |
| 4-Pyridoxicacid | 106.9 | 109.0 | 105.7 | 115.9 | 87.0 | 152.0 |
| FolicAcid | 98.0 | 94.5 | 93.3 | 104.1 | 91.3 | 59.1 |
| 5-MeTHF | 100.4 | 72.2 | 35.5 | 4.3 | 4.6 | 8.8 |
| p-ABGA | 96.2 | 101.4 | 118.2 | 119.1 | 108.1 | 123.5 |
Table 4: Cytotoxic activity of the volatile oil and total extract of Salvia macrosiphon.
Presence of polyglutamate forms in BM
To evaluate the presence of folate polyglutamates in BM, sample preparation included deconjugase treatment. Temperature and incubation time were adapted from previous experiences on food products. Rat serum volume was evaluated by applying the procedure to commercially available standard (5-Me THF diglutamic acid) at various concentrations (data not shown). Samples with big dynamic range (>factor 2) may be affected with rat serum treatment. However, at 1 to 5 ng/mL concentration conversion efficiency seemed to be similar (67% versus 64%). In terms of rat serum volumes, 10 μL seem to be sufficient to cleave almost all diglutamic acid units. Optimal conditions were defined as incubating BM samples after α-amylase treatment with 10 μL of rat serum during 2 hours at 37°C. BM samples were then treated with and without deconjugase. None of the B9 derivative concentration significantly increased after deconjugase treatment. This suggests that 5-Me THF polyglutamate amounts in BM are negligible. However, we may suggest that the presence of antioxidants in the extraction media was desirable when extraction involves longer incubation times as published earlier [38].
Application to BM samples
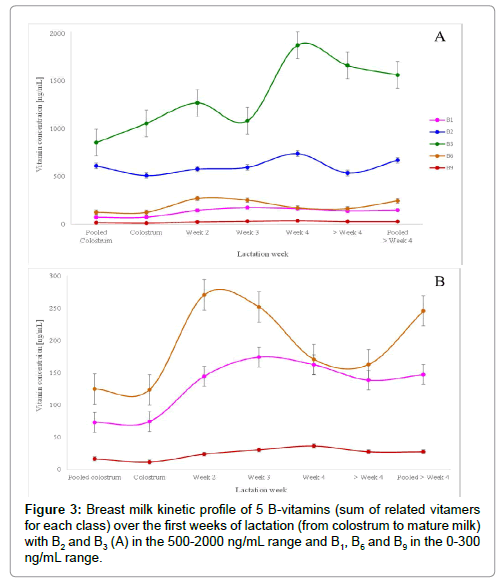
BM samples underwent the sample preparation process and were quantified with the built-in calibration curves (prepared with pooled BM). Each vitamer was quantified individually with its dedicated calibration curve and then concentrations were summed up to obtain the final concentration of each B-vitamin. Figure 3 highlights the evolution of each of the above listed B-vitamin along the 4 first weeks of lactation.
In the early 2000, thiamine concentration was reported as vitamin B1 level in breast milk [15,39]. However, in the recent years, the sum of thiamine and TMP seems to best represent total B1 vitamin levels in breast milk [21,30,40] which was also applied in our work as both compounds were quantified in our work. Indeed, the main secreted form of B1 in breast milk is thiamine monophosphate, contributing for about 60% of total B1 [21,30,40] confirmed in our findings whereas thiamine is the compound used for fortification/supplementation. B1 concentration increases in the transition milk (week 2 to week 4) to reach a steady level around 150 ng/mL in mature milk (>4 weeks).
B2 concentration was obtained by adding riboflavin, FAD and FMN concentrations. Vitamin B2 main secreted form in breast milk is FAD, contributing for at least 60% total B2 [18]. As reported in previous findings [18,39], our results on vitamin B2 emphasized on the importance of quantifying not only riboflavin but also flavin derivatives to best quantify total B2 in breast milk.
Nicotinamide concentration was previously reported as vitamin B3 level in BM [28,39]. Our findings confirm that nicotinamide is the primary B3 vitamer present in BM, however we additionally show that nudifloramide (N-methyl-2-pyridone-5-carboxamide) is also present at a similar level compared to nicotinamide. These findings have never been reported before. We also included nicotinic acid and 1 metabolite nicotinuric acid known to be present in urine [34]. B3 concentration increases in the transition milk (week 2 to week 4) to reach a steady level around 1600 ng/mL in mature milk (>4 weeks).
B6 concentration was obtained by adding pyridoxine, pyridoxamine, pyridoxal, PLP, PMP and 4-pyridoxic acid concentrations. Main secreted form of vitamin B6 in BM was reported to be PL [28,41-44], which has been confirmed in our findings. Among all B6 vitamers, PL contributed to about 75%, PLP to about 15% and only traces for all other forms which is in agreement with previous findings from Hamaker et al. [42,44]. In addition, our findings revealed the presence of 4-pyridoxic acid, which confirms previous findings [45]. Its content reached about 20 ng/mL representing about 5% of total vitamin B6.
B9 concentration was obtained by adding folic acid, 5-Me THF and p-ABGA concentrations. Our results show that the main secreted form was found to be folic acid, contributing to about 75% of total vitamin B9. The presence of polyglutamate forms was investigated but our findings go against previous findings [46]. O’Connor et al. [46] quantified folates by microbiological assay. They applied conjugase treatment to liberate potential polyglutamate forms of folates and quantified total folates after 6 h incubation. The increase in folate amounts in breast milk after deconjugase O’Connor observed is probably due to other existing forms such as 5-formyl THF. In our study, we only focused on the quantification of 5-Me THF in addition to folic acid. Our findings support the recently reported B9 derivative p-ABGA [47] in BM. B9 concentrations triples from week 1 to week 4 to reach a steady level at about 25 ng/mL whereas B2concentration seems to be stable around 600 ng/mL along the first lactation weeks. B6 concentration doubles from week 1 to 2 but decreases afterward.
The calibration ranges described in this paper are comparable to the ones reported previously by Hampel et al. [28] and Ren et al. [29]. Our methodology, despite the use of 200 μL of BM as opposed to only 50 μL used by Hampel et al. [28] and Ren et al. [29], allows for the quantification of TMP, being the main secreted form for B1 evaluation. Measurement of all secreted vitamers will provide a more comprehensive picture of the B-vitamin levels in breast milk to get insight into nutritional needs of the breastfed infants.
Conclusion
This analytical approach based on protein precipitation combined with liquid chromatography-tandem mass spectrometry measurements enabling the accurate quantification of 18 water soluble vitamers in breast milk was successfully developed and validated. Matrix-matched calibration using pooled BM was found to be a reliable tool to provide accurate absolute quantification. This analytical tool can help capture an accurate picture of several water soluble vitamins at one time. This analytical approach demonstrated its application by studying the F/T stability of the B-vitamers as well as quantifying breast milk samples. This allows us to recommend reduced number of freeze/thaw cycles for clinical trial or cohort study samples dedicated to B-vitamin analysis.
Acknowledgement
The authors would like to thank Drs. Laure Poquet, Rachid Bel Rhlid and Chiara Nembrini for the fruitful discussions.
Conflict of Interest
All authors are employees of Nestec. SA, which fully funded the study presented in this manuscript. All authors read and approved the final manuscript.
References
- Elmadfa I, Meyer AL (2012) Vitamins for the first 1000 days: preparing for life. Int J VitamNutr Res 82: 342-347.
- Furdui C, Ragsdale SW (2000) The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J Biol Chem 275: 28494-28499.
- Manzetti S, Zhang J, van der Spoel D (2014) Thiamin function, metabolism, uptake, and transport. Biochemistry 53: 821-835.
- Velasquez-Orta SB (2010) The effect of flavin electron shuttles in microbial fuel cells current production. Appl Microbiol Biotechnol 85: 1373-1381.
- Selhub J (2002) Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging 6: 39-42.
- Clayton PT (2006) B6-responsive disorders: a model of vitamin dependency. J InheritMetabDis29: 317-326.
- Kotloski NJ, Gralnick JA (2013) Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. MBio 4.
- Thakur K (2016) Riboflavin and health: A review of recent human research. Crit Rev Food Sci Nutr.
- Sauve AA (2008) NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther 324: 883-893.
- Wang L (2015) Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. EurJ Nutr 54: 455-464.
- Selhub J (2009) Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. AmJ ClinNutr 89: 702S-706S.
- Czeizel AE (2013) Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients 5: 4760-4775.
- Heseker H (2011) Folic acid and other potential measures in the prevention of neural tube defects. Ann.Nutr Metab 59: 41-45.
- Braegger C (2013) Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr 56: 692-701.
- Ortega RM (2004) Thiamin status during the third trimester of pregnancy and its influence on thiamin concentrations in transition and mature breast milk. BrJ Nutr 92: 129-135.
- Thomas MR (1980) The effects of vitamin C, vitamin B6, vitamin B12, folic acid, riboflavin, and thiamin on the breast milk and maternal status of well-nourished women at 6 months postpartum. Am J Clin Nutr 33: 2151-2156.
- Lonnerdal B, Hernell O (2016) An Opinion on "Staging" of Infant Formula: A Developmental Perspective on Infant Feeding. J Pediatr Gastroenterol Nutr 62: 9-21.
- Roughead ZK, McCormick DB (1990) Flavin composition of human milk. AmJ ClinNutr 52: 854-857.
- Mackey AD, Picciano MF (1999) Maternal folate status during extended lactation and the effect of supplemental folic acid. AmJ ClinNutr 69: 285-292.
- Kang-Yoon SA (1992) Vitamin B-6 status of breast-fed neonates: influence of pyridoxine supplementation on mothers and neonates. AmJ ClinNutr 56: 548-558.
- Coats D (2013) Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. AmJ ClinNutr 98: 839-844.
- Santos J (2012) Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J ChromatogrA 1261: 179-188.
- Leporati A (2005) Application of a liquid chromatography tandem mass spectrometry method to the analysis of water-soluble vitamins in Italian pasta. Analytica chimica acta 531: 87-95.
- Gentili A (2008) Simultaneous determination of water-soluble vitamins in selected food matrices by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun.Mass Spectrom 22: 2029-2043.
- Chen P, Wolf WR (2007) LC/UV/MS-MRM for the simultaneous determination of water-soluble vitamins in multi-vitamin dietary supplements. AnalBioanalChem 387: 2441-2448.
- Chen Z, Chen B, Yao S (2006) High-performance liquid chromatography/electrospray ionization-mass spectrometry for simultaneous determination of taurine and 10 water-soluble vitamins in multivitamin tablets. AnalChimActa569: 169-175.
- Midttun O, Hustad S, Ueland PM (2009) Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid CommunMass Spectrom 23: 1371-1379.
- Hampel D, York ER, Allen LH (2012) Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J Chromatogr B Analyt Technol Biomed Life Sci 903: 7-13.
- Ren XN (2015) Application of UPLC-MS/MS Method for Analyzing B-vitamins in Human Milk. Biomed Environ Sci 28: 738-750.
- Stuetz W (2012) Micronutrient status in lactating mothers before and after introduction of fortified flour: cross-sectional surveys in Maela refugee camp. EurJ Nutr 51: 425-434.
- Meisser Redeuil K (2015) Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J Chromatogr A 1422: 89-98.
- Tamura T, Picciano MF (2006) Folate determination in human milk. J Nutr SciVitaminol (Tokyo) 52: 161.
- Ruckert JW, McCormick DB (2007) Handbook of Vitamins.In: Handbook of Vitamins C.
- Liu M (2012) Simultaneous quantification of niacin and its three main metabolites in human plasma by LC-MS/MS. J ChromatogrB AnalytTechnolBiomedLife Sci904: 107-114.
- Hampel D, Allen LH (2016) Analyzing B-vitamins in Human Milk: Methodological Approaches. Crit Rev Food Sci Nutr 56: 494-511.
- Hampel D, York ER, Allen LH (2012) Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. JChromatogrB AnalytTechnolBiomedLife Sci 903: 7-13.
- Hewavitharana AK (2011) Matrix matching in liquid chromatography-mass spectrometry with stable isotope labelled internal standards-is it necessary? J Chromatogr A 1218: 359-361.
- Quinlivan EP, Hanson AD, Gregory JF (2006) The analysis of folate and its metabolic precursors in biological samples. Anal Biochem 348: 163-184.
- Sakurai T (2005) Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. JNutrSciVitaminol(Tokyo) 51: 239-247.
- Stuetz W (2012) Thiamine diphosphate in whole blood, thiamine and thiamine monophosphate in breast-milk in a refugee population. PLoS One 7: e36280.
- Ren X (2015) B-Vitamin Levels in Human Milk among Different Lactation Stages and Areas in China. PLoS One 10: e0133285.
- Hamaker B (1985) Analysis of B-6 vitamers in human milk by reverse-phase liquid chromatography. AmJClinNutr.
- Kirksey A, Borschel MW (1990) Distribution of B-6 vitamers in human milk during a 24-h period after oral supplementation with different amounts of pyridoxine. AmJ ClinNutr 51: 1062-1066.
- Vanderslice JT (1983) Forms of vitamin B6 in human milk. Am J Clin Nutr 37: 867-871.
- Yagi T (2013) Contents of all forms of vitamin B6, pyridoxine-beta-glucoside and 4-pyridoxic acid in mature milk of Japanese women according to 4-pyridoxolactone-conversion high performance liquid chromatography. J Nutr SciVitaminol (Tokyo) 59: 9-15.
- O'Connor DL, Tamura T, Picciano MF (1991) Pteroylpolyglutamates in human milk. AmJ ClinNutr 53: 930-934.
- Alvarez-Sanchez B (2010) Automated determination of folate catabolites in human biofluids (urine, breast milk and serum) by on-line SPE-HILIC-MS/MS. J Chromatogr A 1217: 4688-4695.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5066
- [From(publication date):
April-2017 - Jul 08, 2025] - Breakdown by view type
- HTML page views : 4095
- PDF downloads : 971