Perspective Article Open Access
A Novel Investigation about the Thermal Behaviour of Gases under the Influence of IR-Radiation: A Further Argument against the Greenhouse Thesis
Thomas Allmendinger*
Independent Scholar, CH-8152 Glattbrugg/Zürich, Switzerland
- *Corresponding Author:
- Dr. Thomas Allmendinger
CH-8152 Glattbrugg/Zürich, Switzerland
Tel: +41448101733
E-mail: inventor@sunrise.ch
Received date: November 18, 2016; Accepted date: March 23, 2017; Published date: March 27, 2017
Citation: Dr. Thomas Allmendinger (2017) A Novel Investigation about the Thermal Behaviour of Gases under the Influence of IR-Radiation: A Further Argument against the Greenhouse Thesis. J Earth Sci Clim Change 8:393. doi: 10.4172/2157-7617.1000393
Copyright: © 2017 Thomas Allmendinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Besides a critical discussion of the greenhouse thesis referring to its basic assumptions, a condensed report of [1] is given here. In contrast to the usual spectroscopic methods, the temperature of a gas embedded in a tube was measured, and not the intensity loss of the radiation. The starting point was to compare air with carbon-dioxide. Moreover, not medium-wave heat radiation was used but, initially, incident solar radiation and, subsequently, artificial radiation from an IR-bulb. In order to minimize the interference by the tube, light-weight building materials were used, such as Styrofoam, transparent plastic foils and aluminium foils. Surprisingly, and in contrast to the expectations, a considerable temperature enhancement could be detected being similar in both cases. The measuring apparatus was improved, and further gases were studied, in particular noble gases. In each case, a temperature increase was detected up-to a limiting value. While the warming-up rate was independent of the gas type, the limiting temperature turned out to be gas-specific, being almost equal for air, pure carbon-dioxide and argon whereas the light gases neon, and in particular helium, exhibited significant lower limiting temperatures. Applying the kinetic gas theory, and assuming a direct correlation between limiting temperature and radiative emission power, a stringent dependency of the product on mean kinetic energy and collision frequency could be deduced. Moreover, the adsorption degree could be calculated, turning out to be very low. The absorption was assumed as a result of vibration of the atomic electron shell, induced by the electromagnetic waves. Comparing the results in sunlight to those obtained in artificial light, the effective wavelength could be assessed delivering the value of 1.9µm. Since the results could be approved by numerous experiments, there is no doubt that a new effect has been discovered which allows questioning the greenhouse theory all the more.
Keywords
Absorption of short infrared-radiation; Heat-radiationtube; Incident solar radiation; Kinetic gas theory; Solar-tube; Thermalradiative measurements at gases
Introduction
The starting point of the here referenced research [1] was the generally accepted greenhouse thesis which assumes that the present climate change is mainly due to the observed growing amount of the so-called greenhouse gases in the atmosphere, particularly of carbondioxide – in spite of the fact that, unlike a greenhouse, the Earth atmosphere doesn’t exhibit a transparent roof, and disregarding the fact that simultaneously other global anthropogenic alterations have been taken place, in particular surface darkening due to the urbanisation and the pollution of glaciers leading to a considerable diminution of the albedo.
This idea takes its source in Fourier’s treatise [2] made in 1827, exhibiting no empirical data or physical calculations and experimental data. The first results were delivered by Tyndall in the sixties of the 19th century [3-5], using artificial IR (= infrared) radiation. His photometric apparatus consisted of metallic tubes as gas vessels and Leslie cubes as heat radiation sources, entailing comparatively low temperatures, namely 100°C and lower. In the nineties, Arrhenius continued such measurements [6,7]. He established the greenhouse thesis claiming that, unlike air, carbon-dioxide considerably absorbs infrared-radiation. Thereby we distinguish between near IR (λ = 0.8 – 3μm), emitted at high temperatures (> 1000 K), and medium IR (λ = 3 – 50μm) occurring at lower temperatures as usual thermal radiation, while IR-radiation with larger wavelengths (λ = 50 – 1000μm) is defined as far IR.
As a consequence of the climate change being obvious from the gradual rise of the average global temperature, as well as from the melting of the ice caps and of the glaciers and from the augmentation of weather extremes and storms, atmospheric sciences have gained in importance over the last decades giving rise to numerous publications making it difficult to summarize the essential points, in spite of the existence of text books such as [8-10]. But overall, the greenhouse thesis has been commonly settled even if its theoretical explanation is quite complicated being not intelligible for most people, and, from a scientific point of view, its empiric basis appears poor while several theoretical presumptions are speculative.
Nevertheless, besides the climate change doubters, some serious criticism concerning climate modelling was recently brought up, particularly by that one of Jakob [11]. However, it is solely focussed on the key areas of clouds and precipitation letting the basic assumptions out of considerations. But as will be shown below, there is reason enough to examine the current climate theory, and in particular the greenhouse thesis, regarding fundamental scientific principles and possibly to question the usual assumptions, right before the novel aspect is brought into consideration which is the subject of the here presented research.
The analytic methods applied in climatology were exclusively photometric ones. While the original experiments of Tyndall and of Arrhenius embraced wide IR-wavelength bands, later investigations concerned spectroscopic measurements separating the electromagnetic radiation into discrete wavelengths. Laboratory experiments were supplemented, and increasingly substituted, by field measurements ending in satellite views delivering results which were interfered by several impacts. Thermal measurements have never been made, except those by pyranometers comprising the whole spectrum, so that direct coherences between light absorption and warming-up effects at matter have not been detected yet.
The natural laws which were used for constructing the theory were confined to the temperature law of Stefan-Boltzmann (1), Planck’s distribution law (2), both being solely valid for black bodies, and Beer- Lambert’s absorption law (3), being unequivocally valid solely for visible light, and not compellingly for IR radiation (see below). These laws were often impermissibly generalized and used in an incorrect way leading to wrong conclusions.
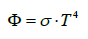 (1)
(1)
σ = Stefan-Boltzmann constant = 5.67â�?�?10-8 Wm-2K-4
T = absolute Temperature [K]
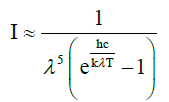 (2)
(2)
λ = wavelength [m] 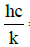 = 0.01439mK
= 0.01439mK
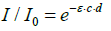 (3)
(3)
ε = absorption coefficient, c = concentration, d = distance
The essential thoughts of the greenhouse theory may be outlined as follows:
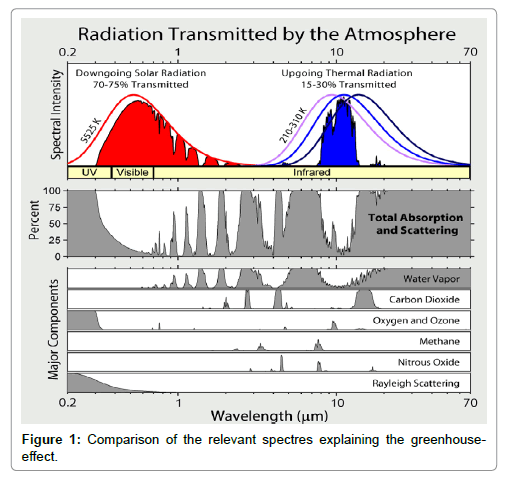
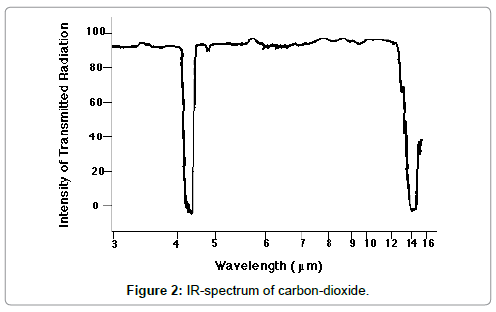
The incoming solar light is partly absorbed by the Earth surface and partly reflected, yielding the surface albedo. Due to this absorption, the surface material of the Earth is warmed-up. Simultaneously, and/or delayed, the warmed Earth surface emits medium-wave IR-radiation which is partly absorbed by the atmosphere, due to the greenhouse gases, and partly emitted into the Space. This emission occurs in a radiation window confined by the absorption behaviour of the greenhouse gases, and the outgoing thermal radiation is detectable outside the atmosphere at satellites. Therefore, according to this model the assumption is made that any warming-up of the atmosphere is exclusively due to a partial absorption of medium-wave IR-radiation while any short-wave IR-absorption can be excluded since it has never been detected spectrometrically. This conception is illustrated in Figure 1 which was been adopted from Wikimedia, while in Figure 2 the spectrum of carbon-dioxide is displayed.
Figure 1: Comparison of the relevant spectres explaining the green house effect. https://commons.wikimedia.org/wiki/File:Atmospheric_Transmission.png.
Against this, at least the following arguments may be alleged:
1. As already found within a previous investigation [12], the greater part – namely at least 60% - of the energy being emitted from a warmed plate to the surrounding atmosphere is transferred by heat conduction, and not by heat radiation obeying Stefan-Boltzmann’s law which is only valid in the vacuum. That part is even enhanced when the air convection is enhanced. Moreover, near the ground the molar concentration of water vapour is much higher than that one of carbondioxide letting assume that its absorbance of heat radiation is much stronger. (e.g. at 20°C and 60% rel. humidity, the molar concentration of water vapour is 36 times larger than that one of carbon-dioxide being 0.038 volume%). Hence it can be assumed that the major part of the heat transfer between Earth surface and atmosphere occurs near the ground [13] while the greenhouse theory neglects that part solely regarding the radiative absorption by CO2 passing the whole atmosphere.
2. Regarding the medium IR-radiation emitted by the Earth surface and detected outside the atmosphere by satellites, several effects have to be anticipated entailing considerable difficulties for a quantitative interpretation by Planck’s law which is solely valid for black bodies. Firstly, the temperature is not distributed uniformly over the whole Earth surface, due to different latitudes, altitudes and temporal delays of the emitters, and not least due to distinct surface albedos (or rather solar absorption coefficients). And secondly, the surface material is variable and heterogeneous, in particular in the case of oceans which exhibit a considerably deviant characteristic. Thus it seems very difficult to estimate the wave-length range which is relevant for the CO2-absorption, letting appear averaging as inadmissible. All the more it’s astonishing that the radiation window widely coincides with the detected radiation peak letting suppose that the absorption is only midget.
3. The Bouger-Lambert-Beer law in its usual version (3) is not identical with Beer’s original approach [14]. It is derived theoretically by integration, assuming that the differential intensity-loss is proportional to the actual intensity which decreases insofar as the intensity decreases, whereas Beer studied the absorption of red light in coloured liquids, using an oil-lamp, as a function of the sample thickness, assuming relation (4):
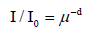 (4)
(4)
μ = Beer’s absorption coefficient, d = thickness (normalized)
Hence the usage of formula (3) appears to be not beyond any doubt. Thereby, the difference between natural and artificial light (i.e., the difference between field- and laboratory-measurements) should be realized, the latter one – as well as the up-going IR-radiation - exhibiting an intensity loss inversely proportional to the distance in the square while the intensity of the incident solar radiation is independent of the distance. Moreover, from organic chemistry is known that IRmeasurements don’t allow quantitative analyses. Anyway, there is no evidence enough for an unconsidered application of this regularity.
4. Between the energetic absorption of electromagnetic radiation by gases and their resulting warming-up no empirical - and also no theoretical - coherence is known which would be needed to carry spectroscopic results onto thermodynamic properties. There is no good reason to assume that absorbed IR-radiation will be entirely transformed into heat. Rather it is conceivable that a part of it is re-emitted, to wit in all directions. But the link between the two phenomena is not known. Aside from that, the small CO2-concentration implicates that approx. the 2500-fold amount of air would have to be cum-warmed.
The absorption of medium-IR can be explained quantummechanically whereby the existence of polar bonds such as O-H (in water) or C=O (in carbon-dioxide) presupposes the interaction with electromagnetic waves leading to vibrations of the atoms which is quantized. So it’s an intra-molecular phenomenon. On the other side, heat is due to the kinetic energy content of the gas which is due to the translation movement of whole molecules or atoms. So it’s an intermolecular phenomenon. That movement is not quantized – as well as the rotation of whole molecules or gases -, and thus cannot be described quantum-mechanically but solely by means of the kinetic gas theory. The latter one is well-known being satisfyingly applied to explain pure heat-conduction in gases, but it was completely disregarded in climatology, yet.
5. The question of radiation emission by hot gases is related with it since it is obvious that any gas, also air, begins to radiate to such an extent as it is warmed-up. This question arises when the socalled radiative energy transfer is studied. But instead of empiric measurements, complicated theories were developed [15-17] starting from the abstruse assumption that the atmosphere behaves like a black body obeying Stefan-Boltzmann’s emission law, and disregarding the kinetic gas theory.
Overall it must be assessed that the atmospheric theory is on a shaky ground widely missing empiric key methods to check the principles and their consequences. Beyond, there is an aspect which hitherto has been overlooked, and which delivers the final proof that the climate theory cannot be true. It is the topic of the here reported author’s work [1] concerning thermal measurements instead of spectroscopic ones, and delivering the evidence that any gas absorbs IR-radiation - but in the short wavelength range -, with the consequence that air is warmedup by direct solar insolation – as well as by artificial IR-light - up to a limiting temperature due to radiative emission, and leading to an equilibrium state.
The defiance of this fact may be apologized by the difficulty of detecting which affords light materials - such as Styrofoam, plastic and aluminium foils - which were not available at the time when the fundamental research was made, but which are needed to avoid interferences of the measuring apparatus with the considered gas. Moreover, conventional quantum mechanics does not deliver a vivid concept to understand radiation absorption by gases, being deterrent because of its difficult intelligibility. However, as could be shown in a further work [18], in case of atomic hydrogen it is possible to visualize three-dimensional wavy electron trajectories in the excited (metastable) states. Analogously, absorption of electromagnetic radiation seems principally possible due to electron jumps, not affording chemical bonds. In fact it is the primary cause for the interaction between light and matter.
However, the here reported observations are not the result of a theoretical expectation but of an unexpected discovery which was possible due to a novel measuring method being created for studying the influence of incident radiation on the thermal behaviour of gases, in particular for searching out the difference between pure air and carbondioxide. Since no significant difference could be detected, further gases were studies, even – and in particular – noble gases. Thereto, instead of often fluctuating sunlight, artificial light under well-defined conditions was used delivering results whose reproducibility and liability could be enhanced by progressive improvement of the apparatus. Finally, a theoretical analysis – not least applying the kinetic gas theory – delivered a self-consistent interpretation approving the accuracy of the observation, even if some uncertainties remain due to the preliminary kind of the investigation, and due to the limited characterization values for the light source.
Concept and Apparatus
Compared to solid bodies, thermal measurements on gases are much more delicate due to their low heat capacity letting suppose a considerable interference of the vessel walls in which the gas is embedded, apart from the fact that gases may move when a temperature gradient arises. Hence, a large ratio between the gas volume and the surface of the vessel must be intended, as well as a low heat capacity of the vessel material. Therefore, it is not surprising that no effect could be detected when erstwhile materials and apparatus were used. But it is all the more astonishing that such measurements have not been made in recent times.
Preliminary tests for the present investigation were made with solar light using square twin-tubes from Styrofoam (3 cm thick, 1 m long, outer diameter 25 cm), each equipped with three thermometers at different positions, and covered above and below by a thin transparent foil (preferably a 0.01 mm thick Saran-wrap). The tubes were pivoted on a frame in such a way that they could be oriented in the direction of the solar light (Figure 3). One tube was filled with air, the other with carbon-dioxide. Incipiently, the tubes were covered on the tops with aluminium-foils being removed at the start of the experiment.
The primary experimental result was quite astonishing in many respects. Firstly: The content gases warmed within a few minutes by approximately 10°C up to a constant limiting temperature. This was surprising - at least in the case of air – for no warming-up was anticipated since sunlight is colourless and allegedly not able to absorb any IR-radiation. However, the existence of a limiting temperature is conceivable since a growing radiative emission has to be expected as far as the temperature rises. Secondly: The limiting temperatures were more or less equal at any measuring point. This means that the intensity of the sun beam was virtually not affected by the heat absorption in the gas tube since the latter one was comparatively weak. And thirdly: Between the two tubes no significant difference could be detected. Therefore, thanks to this simple experiment a special effect of carbondioxide on the direct sunlight absorption could already be excluded.
However, it seemed appropriate to study this effect more precisely with the aim of getting quantitative results, and insight of the theoretically ascertainable coherences. For this purpose, the subsequent experiments were made with artificial light, i.e., with IRlamps, exhibiting a higher amount of IR and being better reproducible, and using a single tube instead of twin tubes (Figure 4). Furthermore, different gases were employed (ambient air, a 4:1 N2/O2-mixture, CO2, Ar, Ne, He from steel cylinders) while the apparatus was improved step by step. Finally, the results obtained in artificial light were compared with the results obtained in solar light with an optimized solar-tube (Figure 5) allowing an approximate statement about the wavelength of the effective radiation. The preparation of the measuring-tube is of great importance since it can influence the reliability of the results. It is explicitly described in [1].
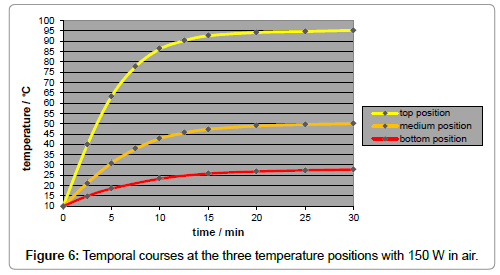
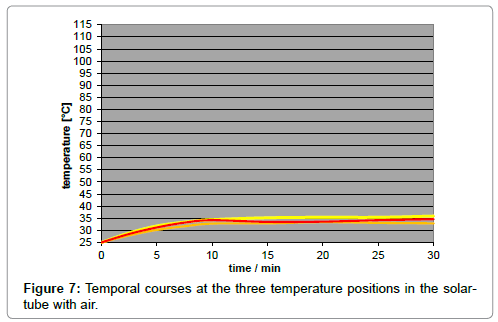
A disadvantage of using artificial light is the inherent temperature gradient along the tube (Figure 6), in contrast to the case of sunlight implying no temperature gradient (Figure 7). It was due to the natural intensity loss, and not to the thermal conductivity of the gas which turned out to be negligible. The local intensity is only approximately appraisable. For improving the accuracy of the results, a minor temperature gradient – or gradient of the limiting temperatures, respectively - was aimed which could be satisfyingly realized by taking several measures such as the mirroring of the walls and of the thermometer contact-tips. As IR-bulbs, “Basking Spots” from ‘exoterra’ (being usual for terraria) were applied in three sizes, according to three intensities (150 W, 100 W, and 50 W), and were inserted into an ‘Arcadia’ reflector. The assessment of their spectral distribution was difficult, leading to the assumption of a peak temperature of approx. 1000 K.
Experimental Results
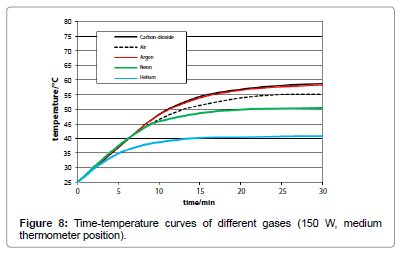
The influence of the several gas kinds was studied by means of artificial IR-light measurements since the reproducibility as well as the temperature enhancement was higher than in the sunlight measurements. Their comparison was made by the respective time/ temperature-curves measured at the medium temperature position, and exhibiting a specific limiting-temperature value. As evident from Figure 8, any gas absorbs IR-light - even the noble gases argon, neon and helium do so - while there is no significant difference between argon and carbon-dioxide, but only a small difference between carbondioxide and air. As separate measurement yielded, no significant difference could be found between ambient air and a pure nitrogen/ oxygen 4:1 mixture. Furthermore, no pressure influence could be detected.
Theoretical Interpretation of the Results
The course of the time/temperature curves can be explained by a linear growth within the initial phase where the gas is continuously warmed-up, on the one hand, and a final constant limiting phase where its radiative emission rate is equal to the warming-up rate, on the other hand. Hence, for determining the absorption degree, solely the linear initial phase has to be regarded, while the values of the limiting temperatures promise to deliver information about the radiative behaviour of the gases.
In order to calculate the heat absorbance of a gas, its molar heat capacity cp is relevant. The multiplication by the measured initial heating rate yields a molar power [W/mole]:
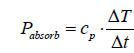 (5)
(5)
whereby, T = temperature [K] or [°C], and t = time [s]
Using for example the values for argon (150 W lamp), yielding a warming-up rate of 20° per 5 min = 0.0667 K/s, and exhibiting a molar heat capacity of 20.85 J/molâ�?�?K, the molar absorption power gets 1.39 W/mole = 0.062 W/l. Assuming at this point a lamp-power of about 120 W, the absorption degree is solely 0.012/mole or 0.00053/l – so it is not surprising, that this effect has so far been overlooked in spectroscopic measurements.
In order to explain the limiting temperature as an implication of the radiative emission, it is necessary to draw on the kinetic gas theory assuming coherence between the emission power and the mean kinetic energy multiplied by the number of collisions per time. When the heating-up rates are equal – as is evident from Figure 5 -, the comparison of two gases yields for the relevant absolute limiting temperatures T1 and T2 the relation
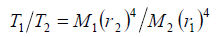 (6)
(6)
Therein M1 and M2 indicate the atomic masses, and r1 and r2 the atomic radii of the compared gases. Furthermore, it is feasible to calculate the collision coefficient ε, defined as
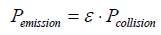 (7)
(7)
yielding the extremely low value of 1.13��?10-12.
Moreover, a rough estimate of the effective wavelength-range is feasible by comparing the absorbance rates due to sunlight and artificial light, as their spectra are not congruent but overlapping, delivering the value of approx. 1.9μm.
Finally, the radiative heat coefficient was determined, i.e., the portion of the radiation energy which is transformed into heat energy. Hereto, the latter one may be calculated using Einstein’s formula E = h·ν = h·c/λ with λ = 1.9μm. Defining the quotient of the two energies as the radiative heat coefficient, the very low value 6.3â�?�?10-5 is obtained, i.e., the amount of radiative energy which is transformed into kinetic heat energy is very small.
Conclusion
Besides a critical discussion of the convenient atmosphere theory profoundly questioning the greenhouse thesis by disclosing several basic errors, the here reported investigation reveals the discovery of direct absorption of shortwave IR-radiation by air. It is part of the incident solar light, but also of artificial light which enables a more exact detection. It is caused by another effect than the one which is responsible for the longer-wave absorption being observed at carbondioxide, and it is not detectable by IR-spectroscopy since its absorption coefficient is too low. However, it is clearly detectable by means of the here applied apparatus leading to a distinct temperature elevation up to a limiting temperature which depends on the radiative emission. The limiting temperature depends on the gas kind, whereby practically no difference between air and carbon-dioxide could be found. However, a significant difference could be found between the noble gases argon and helium, and theoretically explained applying the kinetic gas theory, assuming coherence between the emission power and the mean kinetic energy multiplied by the number of collisions per time. Finally, an estimation of the relevant wave length was made admittedly being only approximate.
The theory is not yet complete. e.g. it doesn’t allow calculating the limiting temperatures absolutely. Moreover, in open air the warmed air immediately arises thereby cooling down and covering up this effect. And finally, in the outdoors, this effect is overlaid by the heat exchange of the atmosphere with the ground which is warmed-up due to solar insulation, and which depends on the albedo of its surface. Indeed, a computable modelling comprising all these effects seems very difficult and hardly feasible.
Nevertheless, that direct absorption effect which was discovered thanks to this method probably contributes significantly to the warmingup of the atmosphere while the warming-up due to carbon-dioxide can be neglected. But since the direct absorption cannot be influenced, the surface albedo must be focussed as the governing factor providing the only opportunity to mitigate the climate, or at least the microclimate, by changing colour and structure of the surface, particularly in urban areas. However, a prediction seems not feasible since the global climate is too complex. But the greenhouse theory turns out to be a phantasm delivering the wrong diagnosis for the climate change, and a wrong diagnosis cannot enable a healing.
Acknowledgement
The present work has been carried out independently but not without the professional support of Dr. Andreas Rüetschi (physicist, from the Swiss Federal Institute of Technology, Zurich).
References
- Allmendinger T (2016) The thermal behaviour of gases under the influence of infrared-radiation. Inter J Phys Sci 11: 183-205.
- Fourier M (1827) Mémoire sur les températures du globe terrestre et des espaces planétaires; Mémoires de l’Académie Royale des Sciences de l’institut de France : 569-604.
- Tyndall J (1861) Thebakerian lecture on the absorption and radiation of heat by gases and vapours, and on the physical connexion of radiation, absorption, and conduction. PhilosophMagaz 22: 169-285.
- Tyndall J (1863) On the radiation through the earth’s atmosphere. PhilosophMagaz 25: 200-206.
- Tyndall J (1872) Contributions of molecular physics in the domain of radiant heat
- Arrhenius S (1896) On the influence of carbonic acid in the air upon the temperature of the ground. Philosophical Magazine 41: 238-276.
- Arrhenius S (1901) Ueber die wärmeabsorption durch kohlensäure. Annalen der Physik 309: 690-705.
- Hartmann DL (1994) Global physical climatology.
- Visconti G (2001) Fundamentals of physics and chemistry of the atmosphere. Springer.
- Boeker E, Van GrondelleS (2011) Environmental physics:Sustainable energy and climate change, third edition.
- Jakob C (2014) Going back to basics. Nature climate change 4: 1042-1045
- Allmendinger T (2016) The solar-reflective characterization of solid opaque materials. Intern J Sci and TechnolEducat Res 7: 1-17.
- Barrett J (1995) The roles of carbon dioxide and water vapour in warming and cooling the Earth’s troposphere. Spectrochimica Acta 51: 415-417.
- Beer A (1852) Bestimmung der absorption des rothen lichts in farbigen flüssigkeiten. Annalen der Physik und der physikalischen Chemie 86: 78-88.
- Tien CL (1968) Thermal radiation properties of gases, in: hartnett jr and irvine t (Eds.). Advances in Heat Transfer 5: 253-324.
- Cess RD, Tiwari SN (1972) Infrared radiative energy transfer in gases. advances in heat transfer 8: 229-283.
- Joseph HL, Wiscombe WJ, Weinman JA (1976) The delta-eddington approximation for radiative flux transfer. J AtmoSci 33: 2452-2459.
- Allmendinger T (2016) A classical approach to the de broglie-wave based on bohr’s h-atom-model. Int. J AppliMathem and Theoret Phys 2: 1-15.
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 6501
- [From(publication date):
March-2017 - Jul 16, 2025] - Breakdown by view type
- HTML page views : 5419
- PDF downloads : 1082