A Novel Human Neuronal Cell Model to Study Iron Accumulation in Parkinson’s Disease
Received: 29-Jan-2019 / Accepted Date: 04-Jan-2019 / Published Date: 11-Feb-2019 DOI: 10.4172/2161-0460.1000461
Abstract
Objectives: With an estimated seven to ten million sufferers worldwide, Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder. Progress in elucidating its causes has been slow, partly due to the lack of human-relevant models. Similarly, while the contribution of iron is increasingly advocated, identifying its role in disease progression remains challenging mainly due to the lack of valid model. In this study, we created Parkinson-like conditions in a human neuron model and conducted preliminary studies on iron-related parameters to
assess whether these cells replicated iron accumulation observed in Parkinsonism.
Methods: ReNcell VM (human neural progenitor) were differentiated into dopaminergic neurons (dDCNs) and treated with neurotoxin 6-hydroxy dopamine (100 μM) to mimic Parkinsonism. Total intracellular, mitochondrial and cytoplasmic iron was measured by ferrozine assay. Expression of iron-related genes TFRC, SLC40A1, HAMP and SLC25A37 were assessed through real-time PCR.
Results: Data showed that the treated dDCNs accumulated iron over time and exceeded levels measured in untreated dDCNs by 2.5-fold at 48 h (p<0.02). Following the treatment, the treated cells showed lower expression of TFRC (p<0.05), but substantially higher mRNA expressions of SLC40A1 (9-fold; p<0.02) and HAMP (5.7-fold; p<0.05), along with higher intracellular iron (p<0.05). Higher iron accumulation in the mitochondria than cytosol (p<0.05), was also observed with increased expression of the mitochondrial iron-importer SLC25A37 (p=0.08).
Conclusion: Our Parkinsonian model demonstrates iron accumulation and elevated HAMP expression as previously described in PD phenotype. The observed mitochondrial iron shuttling, which is proposed to be one of the primary contributors of oxidative stress in PD, calls for further investigation. The differences observed in distribution of iron in our human model and with the expression of major iron-related proteins, indicate that our model reproduces the disease state successfully, and suggests that further study could help in advancing our understanding of PD.
Keywords: Iron; Parkinson; Dopaminergic; Mitochondrial iron; Hepcidin; Neurons
Introduction
With millions of sufferers world-wide, Parkinson’s disease (PD) is characterized by the gradual death of dopamine- producing neurons, which clinically manifests in impaired motor function and mobility. In the advanced stage, it progresses to dementia and presents a wide spectrum of neuro-psychiatric problems [1]. Presently, treatment with the dopamine-precursor L-DOPA is widely used to alleviate the symptoms of the disease. Unlike dopamine, this can cross the blood brain barrier [2], but it does not rescue the continuous loss of dopaminergic neurons, and eventually fails to serve its purpose in the advanced stage of the disease. So far, there is no cure for PD; a major hindrance being the inability to identify the cause of death of dopaminergic neurons.
A hallmark of PD is the selectively increased iron deposits in the substantia nigra pars compacta (SNPC), a region in the mid-brain [3]. Such accumulation of iron in the brain of PD patients is well established [4]. This unexplained elevation in the labile iron pool is believed to participate in oxidative damage and iron deregulation, which contributes towards dopaminergic degeneration [5]. Whereas in the haemochromatotic patients, a 10-20-fold increase in iron stores seem to be necessary before clinical manifestations are observed, only a 2-fold increase in iron content in SNPC of PD patients have been proposed to be sufficient for disease progression [5]. While this highlights the significance of iron content variation in the brain, the severity of its impact and the progression of PD in human are incompletely understood. This lack of understanding is partly due to the lack of human-relevant models, with the majority of studies being conducted on animal models that are unable to fully mimic the human PD pathophysiology.
Hence, in this short preliminary study, we aimed to create Parkinsonlike conditions in a human neuronal cell system and analyze iron-related parameters in these cells to assess whether the cells resembled iron accumulation, as observed under Parkinsonian conditions and thereby evaluate whether these cells would be suitable for further iron-related studies on Parkinsonism. In addition, we aimed to examine the pattern of subcellular iron accumulation.
Accordingly, human neuronal progenitor cells were differentiated into dopaminergic neurons and then treated with 6-hydroxy dopamine (6-OHD). Total cellular iron accumulation was measured at different time points. Crucial iron-related genes were examined; HAMP (gene encoding the iron-hormone hepcidin), TFRC (encoding cellular iron-uptake protein transferrin receptor 1), SLC40A1 (encoding cellular iron-exporter protein ferroportin) and SLC25A37 (mitochondrial ironimporter protein mitoferrin-1). The relative iron distribution in cytosol and mitochondria was assessed. Comparisons were drawn between differentiated dopaminergic neurons (untreated control) and 6-OHDtreated dopaminergic neurons.
Methods and Materials
Cell culture
ReNCell VM (Millipore, UK), the human neural progenitor cells were supplemented with 20 ng/ml epidermal growth factor (EGF) and 20 ng/ml basic fibroblast growth factor (bFGF) and maintained in serum-free ReN cell NSC maintenance medium (Millipore, UK).
Treatments
The cells were differentiated into dopaminergic neurons (dDCNs) (untreated control cells). Then, these cells were treated with 100 μM 6-OHD for 2 hours (h) to induce oxidative stress and create Parkinson-like conditions, as described previously [6]. After this, cell culture medium was replaced with fresh medium, and cells were either harvested immediately (0 h) or left in incubator at 37oC and harvested at different time intervals. These cells are referred as treated cells in this study. Mitochondrial and cytosolic fractions were isolated using mitochondrial isolation kit (Thermo Scientific, USA), as per manufacturer’s instructions and as described previously [6]. Essentially, cells were collected and pelleted at 850 X g for 2 min. Reagent A was added to cells. Following incubation on ice for 2 minutes, reagent B and reagent C (1/100) were added and the samples were centrifuged at 700Xg for 10 min at 4oC. The supernatant was centrifuged again at 12,000Xg for 15 min at 4°C. This supernatant was the cytosol fraction. The pellet was treated with reagent C and centrifuged at 12,000Xg for 5 min. This was the mitochondrial fraction. To ensure the purity of cytosolic and mitochondrial fractions, western blot analyses using 50 microgram proteins isolated from control cytosolic and mitochondrial fractions were carried out as described previously [6]. The following specific marker antibodies were used: mouse monoclonal anti-actin (a cytosolic marker, 1: 1000; Abcam, Cambridge, UK) and rabbit monoclonal anti-cyto- chrome C (a mitochondrial marker, 1: 1000; Millipore).
Measurement of iron content
Iron content was determined by the ferrozine assay and expressed as nmoles of iron per mg of protein quantified by the Bradford method, as previously performed [7-9]. The ferrozine assay can detect low amounts of ferrous and ferric iron from 2 to 300 μM [10]. It has been previously used by other groups to measure intracellular iron content in various cell types including cultured brain astrocytes [10] and HepG2 cells [11].
Gene expression analysis
Primers (Invitrogen, UK) for gene expression analyses of HAMP, TFRC and SLC40A1 were as previous described [7]. Primers TAGCCAACGGGATAGCTGG (F) and GTGGTGTAGCTCCGGTAGAAG (R) were used to assess SLC25A37 expression (12). RNA extraction, cDNA preparation, real-time PCR and data analyses were conducted as previously described [7-9]. Essentially, RNA was extracted by using the TRI reagent (Sigma-Aldrich, UK), and cDNA was synthesised using QuantiTect reverse transcription kit (Qiagen, UK). Gene expression was analysed through real-time PCR by using Qiagen’s Quantifast SYBR green kit in Rotor-gene Q machine (Qiagen, UK). Data was obtained and analysed by using Rotor-gene software series 1.7, and was expressed as fold expression change, inferred by 2-ΔΔCt.
Statistical Analyses
Data analysis was performed using Student’s T-test. The level of significance was set at p<0.05. Data was presented as mean ± SEM.
Results
Cells accumulated iron over time
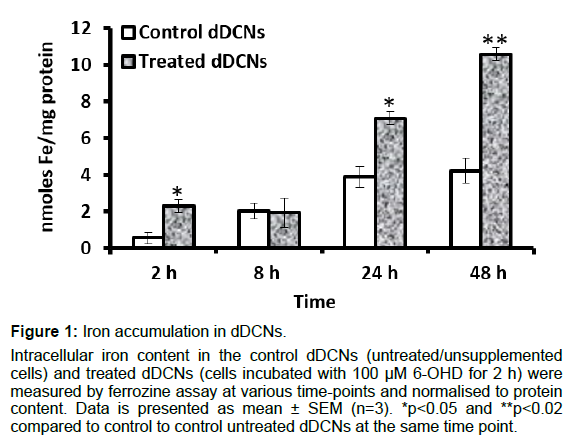
The control dDCNs (untreated cells) showed subtle iron accumulation up to 24 h and plateaued thereafter (Figure 1). The treated cells (cells treated with 100 μM 6-OHD for 2 h) gradually accumulated iron over time and exceeded levels in the control cells by approximately 1.8-fold (p<0.05) at 24 h and 2.5-fold (p<0.02) at 48 h (Figure 1). As the study involved assessing cellular iron accumulation, the concentration of iron in the maintenance medium was measured and determined as approximately 4 μM (supplementary Figure 1).
Figure 1: Iron accumulation in dDCNs.
Intracellular iron content in the control dDCNs (untreated/unsupplemented cells) and treated dDCNs (cells incubated with 100 µM 6-OHD for 2 h) were measured by ferrozine assay at various time-points and normalised to protein content. Data is presented as mean ± SEM (n=3). *p
Low mRNA expression of iron-importer, and high expression of iron-exporter and iron-regulator
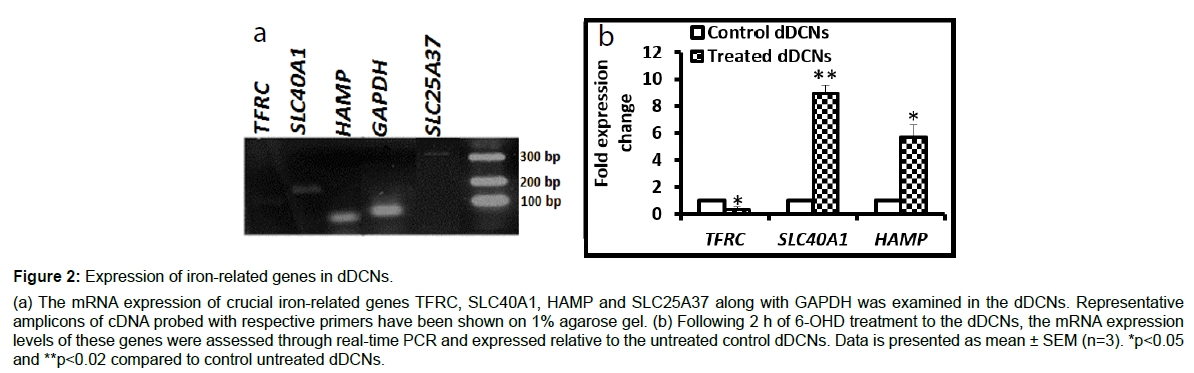
The mRNA expression of major (iron-related genes following 2 h of 6-OHD treatment) was confirmed on 1% agarose gel (Figure 2A). Compared to the untreated control dDCNs, the treated cells showed significantly lower mRNA expression of the iron-importer gene TFRC (p<0.05), along with substantially higher expression of the iron-exporter gene SLC40A1 (9-fold, p<0.02) and iron-regulator gene HAMP (5.7- fold, p<0.05) (Figure 2B).
Figure 2: Expression of iron-related genes in dDCNs.
(a) The mRNA expression of crucial iron-related genes TFRC, SLC40A1, HAMP and SLC25A37 along with GAPDH was examined in the dDCNs. Representative amplicons of cDNA probed with respective primers have been shown on 1% agarose gel. (b) Following 2 h of 6-OHD treatment to the dDCNs, the mRNA expression levels of these genes were assessed through real-time PCR and expressed relative to the untreated control dDCNs. Data is presented as mean ± SEM (n=3). *p
Cells accumulated iron in the mitochondria
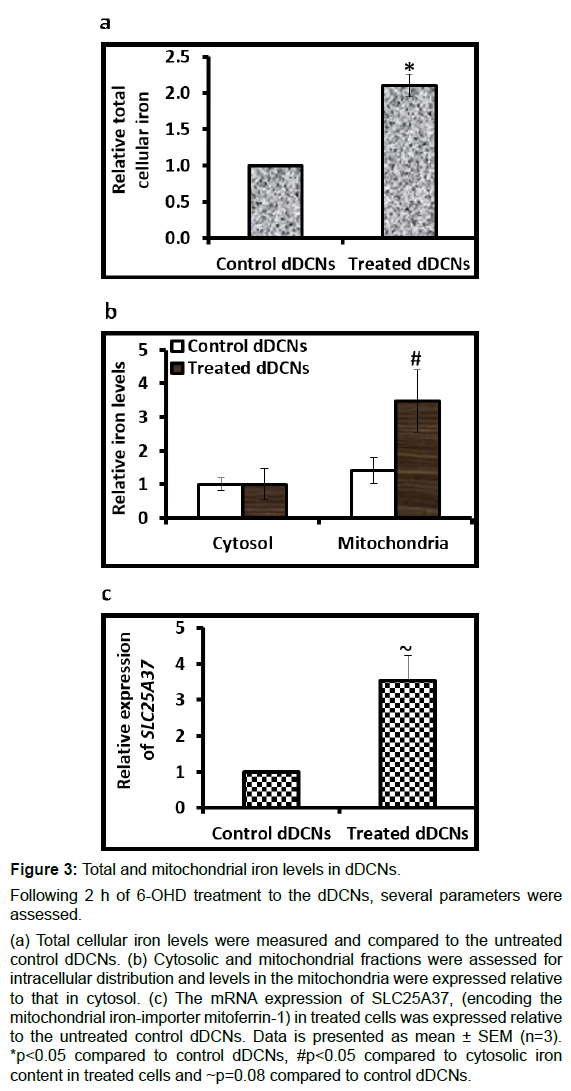
The treated cells showed 2.1-fold (p<0.01) higher total cellular iron levels: Than the control cells (Figure 3A). While the control cells showed no major difference in iron content between the mitochondria and the cytosol, the treated cells showed significantly higher levels of iron in the mitochondria than the cytosol (p<0.05) (Figure 3B). Moreover, expression of the mitochondrial ironimporter gene SLC25A37 was 3.5-fold (p=0.08) higher in the treated cells than untreated cells (Figure 3C).
Figure 3: Total and mitochondrial iron levels in dDCNs.
Following 2 h of 6-OHD treatment to the dDCNs, several parameters were assessed.
(a) Total cellular iron levels were measured and compared to the untreated control dDCNs. (b) Cytosolic and mitochondrial fractions were assessed for intracellular distribution and levels in the mitochondria were expressed relative to that in cytosol. (c) The mRNA expression of SLC25A37, (encoding the mitochondrial iron-importer mitoferrin-1) in treated cells was expressed relative to the untreated control dDCNs. Data is presented as mean ± SEM (n=3). *p
Discussion
Iron accumulation in the brain is common in several neurodegenerative diseases including Parkinson’s disease [5,12,13]. Indeed, excess iron accelerates the Fenton reaction to generate noxious free radicals that cause cellular and tissue damage and thereby exacerbates disease pathology. However, iron regulation in the brain is poorly understood and whether increased brain iron is the cause or consequence of the pathology remains to be elucidated. In PD-related studies, the challenge arises particularly due to the lack of appropriate models that can accurately mimic human PD pathophysiology. Hence, this short preliminary study aimed to confirm iron accumulation in this human cell system of PD, so that future iron-related studies could be conducted using these cells to better understand human PD pathology.
Following the neurotoxin treatment on differentiated dopaminergic neurons, cellular iron accumulation was examined over time. In the treated cells, the gradual increase in iron accumulation over time (Figure 1) (1.8-fold and 2.5-fold higher than control cells at 24 h and 48 h, respectively) is physiologically relevant [5] and demonstrate cellular iron retention and reflect a typical PD phenotype. This suggests that these cells could be used in future iron-related studies to understand PD pathology. Notably, although, the iron concentration of the maintenance medium was only 4 μM (Supplementary Figure 1), which is 2.5-fold lower than the serum iron concentration in human blood (10-30 μM) [14], there was a substantial increase in intracellular iron accumulation in the treated cells (Figure 1). As the maintenance medium was devoid of both, serum and transferrin, transferrin-bound-iron uptake was improbable, suggesting that the iron uptake was probably due to nontransferrin bound iron (NTBI). Unlike the tightly regulated process of neuronal iron-uptake via transferrin [15], NTBI uptake is unregulated and leads to excess cellular iron deposition in several iron-related pathologies such as hereditary hemochromatosis [16]. In this study, indication of increased NTBI uptake in the treated cells suggests that under Parkinsonian conditions, the neurons may acquire the ability to uptake increased amount of NTBI, thereby enhancing oxidative stress, as observed in mice and rat neurons [17,18]. Nevertheless, it would be interesting to determine the rates of iron uptake by examining TFRC expression along with NTBI uptake and comparing between cells and between time points. In tandem with this, it would also be interesting to study the alterations in iron export genes and proteins. This is beyond the scope of this preliminary study and calls for further investigation.
We then examined the iron content and the expression of selected iron-related genes immediately following the neurotoxin treatment. In our knowledge, for the first time, we confirmed the mRNA expressions of the iron-transporters TFRC, SLC40A1 and SLC25A37, and the iron-hormone HAMP in these cells (Figure 2A). Comparisons in gene expression were made between the untreated and treated cells (Figure 2B). Lower expression of the iron-importer gene TFRC, together with increased expression of the iron-exporter gene SLC40A1 is indicative of intracellular iron excess [19] (Figure 2B). The elevation in HAMP mRNA expression in the treated cells (Figure 2B) is in line with increased hepcidin expression observed in the ageing brain and in rat models [20]. Here, it could be due to elevated cellular iron levels (Figure 3A), as hepcidin is responsive to increased iron levels [21]. However, raised HAMP expression could also be due to inflammation from 6-OHD treatment, as hepcidin is also produced under inflammatory conditions [22]. Moreover, elevated hepcidin expression along with reduced TFRC expression (Figure 2B) in the initial stage could be a protective response against neuronal iron accumulation, as described by other groups [23]. Under physiological conditions, increased HAMP expression in the brain is highly probable due to either or both the aforementioned conditions because the PD patients demonstrate both, high iron deposition as well as inflammation [24].
Mitochondria are sites for redox reactions, haem biogenesis [25] and iron-sulphur clusters [26]. Thus, iron accumulation in this compartment is particularly prone to oxidative damage via Fenton’s reaction [27]. Therefore, not only cellular iron acquisition but also the subsequent iron distribution into cellular organelles plays a significant role in disease pathology. In our cell system, cytosolic and mitochondrial fractions were examined. Data indicated iron accumulation in the mitochondria (Figure 3B), which was further supported by an increased expression of the mitochondrial iron-uptake gene SLC25A37 (Figure 3C). A similar iron distribution pattern could be expected in human PD, where increased mitochondrial iron uptake, may lead to overwhelming oxidative stress and exacerbate PD pathology. Our data and the resulting conclusion is in line with other studies which show that mitoferrin1- (encoded by SLC25A37) is capable of transporting iron into the mitochondria of nonerythroid cells [28]. Note that major iron elevations (iron loading) in the mitochondria, should not be expected as the cells were in maintenance medium without any additional supplementation of iron.
These preliminary studies should be followed by subsequent proteomic studies in these cells to examine levels of iron-transporters at various time points. Study of iron-importer protein and iron-shutting in the mitochondria should be prioritized.
Conclusion
Increased iron content accelerates the pathological progression of several neurological conditions, where the exact role of iron remains unknown. Particularly with PD, the major obstacle is the lack of humanrelevant models that would accurately mimic human pathophysiology. Herein, 6-OHD-treated human dDCNs displayed Parkinsonian characteristics of gradual cellular iron accumulation over time and elevated HAMP expression. Also, the cells expressed other important iron-related genes such as the cellular iron-importer TFRC, cellular ironexporter SLC40A1 and mitochondrial iron-importer SLC25A37. Increased mitochondrial iron accumulation suggests further studies to clarify mitochondrial iron regulation in PD, as this could be the major cause of oxidative stress and consequent PD pathology. These exciting results suggest the suitability of this cell model for further iron-related studies on PD. Further studies will be very useful in elucidating PD mechanisms and will greatly aid in devising therapeutic strategies to halt and prevent the iron-induced oxidative damage in dopaminergic cells.
References
- Chinta SJ, Andersen JK (2005) Dopaminergic neurons. Int J Biochem Cell Biol 37: 942-946.
- Jankovic J, Aguilar LG (2008) Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat 4: 743-757.
- Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB (1991) Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem 56: 978-982.
- Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, et al. (2000) The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20: 6048-6054.
- Crichton RR, Dexter DT, Ward RJ (2011) Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm (Vienna) 118: 301-314.
- Ahmed BY, Husnain O, Stafford R, Howard M, Gujar AS, et al. (2013) Hyperphosphorylation of CREB in human dopaminergic neurons: A kinetic study of cellular distribution of total CREB and phospho-CREB following oxidative stress. Neuroreport 24: 757-762.
- Mehta K, Busbridge M, Renshaw D, Evans RW, Farnaud S, et al. (2016) Characterization of hepcidin response to holotransferrin in novel recombinant TfR1 HepG2 cells. Blood Cells Mol Dis 61: 37-45.
- Mehta K, Greenwell P, Renshaw D, Busbridge M, Garcia M, et al. (2015) Characterisation of hepcidin response to holotransferrin treatment in CHO TRVb-1 cells. Blood Cells Mol Dis 55: 110-118.
- Mehta KJ, Farnaud S, Patel VB (2017) HFE mRNA expression is responsive to intracellular and extracellular iron loading: short communication. Mol Biol Rep 44: 399-403.
- Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331: 370-375.
- Huang X, Dai J, Fournier J, Ali AM, Zhang Q, et al. (2002) Ferrous ion autoxidation and its chelation in iron-loaded human liver HepG2 cells. Free Radic Biol Med 32: 84-92.
- Hung HI, Schwartz JM, Maldonado EN, Lemasters JJ, Nieminen AL (2013) Mitoferrin-2-dependent mitochondrial iron uptake sensitizes human head and neck squamous carcinoma cells to photodynamic therapy. J Biol Chem 288: 677-686.
- Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, et al. (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68: 1820-1825.
- Cook JD, Flowers CH, Skikne BS (2003) The quantitative assessment of body iron. Blood 101: 3359-3364.
- Connor JR, Menzies SL (1995) Cellular management of iron in the brain. J Neurol Sci 134 Suppl: 33-44.
- Pietrangelo A (2010) Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 139: 393-408.
- Garrido-Gil P, Rodriguez-Pallares J, Dominguez-Meijide A, Guerra MJ, Labandeira-Garcia JL (2013) Brain angiotensin regulates iron homeostasis in dopaminergic neurons and microglial cells. Exp Neurol 250: 384-396.
- Bishop GM, Dang TN, Dringen R, Robinson SR (2011) Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox Res 19: 443-451.
- Muckenthaler MU, Galy B, Hentze MW (2008) Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 28: 197-213.
- Vela D (2018) Hepcidin, an emerging and important player in brain iron homeostasis. J Transl Med 16: 25.
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, et al. (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276: 7811-7819.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, et al. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101: 2461-2463.
- Du F, Qian ZM, Luo Q, Yung WH, Ke Y (2015) Hepcidin Suppresses Brain Iron Accumulation by Downregulating Iron Transport Proteins in Iron-Overloaded Rats. Mol Neurobiol 52: 101-114.
- Xu Q, Kanthasamy AG, Jin H, Reddy MB (2016) Hepcidin plays a key role in 6-OHDA induced iron overload and apoptotic cell death in a cell culture model of Parkinson's disease. Parkinsons Dis 2016: 8684130.
- Ajioka RS, Phillips JD, Kushner JP (2006) Biosynthesis of heme in mammals. Biochim Biophys Acta 1763: 723-736.
- Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, et al. (2012) The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823: 1491-1508.
- Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord 18 Suppl 1: S210-212.
- Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, Kaplan J (2009) Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol Cell Biol 29: 1007-1016.
Citation: Mehta KJ, Ahmed BY, Farnaud SJC (2019) A Novel Human Neuronal Cell Model to Study Iron Accumulation in Parkinson’s Disease. J Alzheimers Dis Parkinsonism 9: 461. DOI: 10.4172/2161-0460.1000461
Copyright: © 2019 Mehta KJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5202
- [From(publication date): 0-2019 - Oct 31, 2025]
- Breakdown by view type
- HTML page views: 4310
- PDF downloads: 892