Research Article Open Access
A Novel Fully Automated Incubation, Manipulation and Documentation System for the Avian Embryogenesis
Mohrhardt E1, Schmitt D1, Gorjup E1, Howitz S2, Zimmermann H1,3 and Fuhr G1,3*1Fraunhofer Institute for Biomedical Engineering, Ensheimer Strasse 48, 66386 St. Ingbert, Germany
2GeSiM mbH, Bautzner Landstraße 45, 01454 Grosserkmannsdorf, Germany
3Saarland University, Campus Saarbrücken, 66123 Saarbrücken, Germany
- Corresponding Author:
- Fuhr G
Fraunhofer Institute for Biomedical Engineering
Ensheimer Strasse 48, 66386 St. Ingbert, Germany
Tel: +49 (0) 6894/980-291
E-mail: elmar.mohrhardt@ ibmt.fraunhofer.de
Received date: May 12, 2015; Accepted date: September 25, 2015; Published date: September 30, 2015
Citation: Mohrhardt E, Schmitt D, Gorjup E, Howitz S, Zimmermann H, et al. (2015) A Novel Fully Automated Incubation, Manipulation and Documentation System for the Avian Embryogenesis. J Biotechnol Biomater 5:202.doi:10.4172/2155-952X.1000202
Copyright: © 2015 Mohrhardt E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Scientifically the avian egg is a well-established and increasingly used animal model predominantly represented by the chicken (gallus gallus domesticus). Apart from developmental biology studies, chicken eggs are used in angiogenesis, tumor and irritation studies. For all those experimental setups a culture and incubation system is required providing excellent documentation of environmental parameters as temperature and relative humidity and optical accessibility, flexible manipulation during the complete embryonic development of the chicken (usually 21 days) and stable survival rate. Here we present an avian egg incubator with a modular lid system comprising a glass window with condensation prevention and a parallel access for manipulation. We show the influence of light exposure as well as turning speed and frequency on the embryo survival rate for optimized culture conditions. Incubator and lid system were evaluated successfully up to the hatch of normally developed chicken, while automatically documenting their embryogenesis. The new incubation system holds benefits like automated high quality documentation, online manipulation and adjustable incubation and opens up completely new applications in the field of avian embryo culture.
Keywords
Novel avian incubator; Automated high quality optical documentation; Online manipulation; Time lapse imaging; Bioreactor; Recipient eggshell culture; Avian embryogenesis
Introduction
With Aristotle’s studies on the chicken’s embryonic development the avian egg became the first model organism for scientific use [1]. Later, the chicken embryogenesis was the key subject in the dispute between preformationists and epigenesists for many centuries [2]. In 1951 Hamburger and Hamilton provided an important tool by describing the single stages of the chicken embryogenic development very detailed, allowing accurate staging of the whole development [3]. In the field of developmental biology the chicken is still used as the model organism for birds. Beside this the chicken embryo is a frequently used organism for applications like angiogenesis [4,5], tumorgenesis [6,7] and irritation assays [8,9]. The latter is known as hen’s egg test on chorionallantoic membrane (HET-CAM) using the highly vascularized chicken chorionallantoic membrane (CAM). The irritation potential of chemicals, formulations [9] and pesticides [10] is tested with the HET-CAM assay evaluating the reaction of the applied substances on the vascularized CAM. It was developed to replace the Draize test in the rabbit eye by Lüpke [8]. A further example for the scientific use of the chicken embryo is the generation of transgenic animals [11]. Here, the cells of the germinal disk are genetically manipulated to produce e.g. therapeutically relevant proteins in the laid eggs of the transgenic chicken.
For the experimental use the egg has to be opened to access the embryo and the vascularized CAM. Subsequently, the embryo is either cultivated in its natural eggshell (in ovo) or in artificial vessels (ex ovo), the former being the more common approach. Here, the shell is half opened to reach the embryonic tissues and the opening is sealed after manipulation or documentation to prevent desiccation and contamination. The first long term ex ovo cultivation system for the chicken has been established in the early 1970s by Elliott and Bennett [12] who cultured the embryos in polyethylene bags for 10-12 days. In 1974 Auerbach et al. published a system cultivating chicken embryos in a petri dish (10 cm diameter) for a longer period (up to day 20) [13]. Having many advantages like a big CAM surface for the different tests this is one of the most frequently used cultivation systems for chicken embryos today. However, due to the unnatural shape of the petri dish and the impossibility of hatching the chicks, the Auerbach system is not sufficient for applications like the production of transgenic chicken. In 1988, Perry first reported a culture system for the chicken embryo from the oviduct to the hatching. Three different eggshell systems and egg transfers were used for the different periods of the embryogenesis [14]. These culture systems are closed with cling film to prevent desiccation and contamination. This approach shares the main positive characteristics of the Auerbach system additionally providing a high hatching rate. With the development of new applications like tissue culture on the CAM with more complex setups the requirements for the culture systems rose [15,16]. For those setups, there is a need for combining optimal optical access and high-quality imaging quality for online documentation with a stable culture. Today, no system is known to meet these requirements. Here, we developed a semi-artificial incubation system that fulfills these characteristics and allows embryonic development up the hatch of the animal. Additionally it provides features like compactness, autarky and manipulation possibilities.
Materials and Methods
Incubation of fertilized eggs
Fertilized chicken eggs (Lohmann Selection Longhorn) were obtained from LSL Geflügelvermehrungsbetrieb in Dieburg, Germany. After cleaning the eggs they were weighed and stored in a fridge (Lovibond Thermostatschrank ET 619-4 Typ 180, Liebherr, Bulle, Switzerland) at 16°C for at least 24 hours and no longer than 5 days. Damaged eggs were discarded. The standard breeding was done in a motor breeding incubator (standard incubator, Top-Profi 300, J. Hemel Brutgeräte GmbH and Co. KG, Verl-Kaunitz, Germany). The eggs were placed blunt end up in the incubator at 37,5°C and a relative humidity of 65%. They were turned every 2 hours to an angle of 30° with a turning speed of 0,6°/s. For hatching, on day 18 of incubation, the eggs were placed in a Hatcher (Bruja Modell 168/D, Brutmaschinen-Janeschitz GmbH, Hammelburg, Germany) where they were kept at 37,5°C and 80% relative humidity without turning and no further manipulation. Experiments were conducted in accordance to the Guide for the Care and use of Agricultural Animals in Research and Teaching [17]. Unnecessary discomfort to embryos and animals were avoided.
Preparation and incubation of the recipient eggshell culture
All procedures concerning the preparation of the cultures were performed under sterile conditions in a laminar flow (Herasafe KS18, Kendro Laboratory Products GmbH, Langenselbold, Germany). For the recipient eggshell an egg, 25 g heavier as the fertilized egg (Geflügelhof Vogelgesang, Mandelbachtal-Ommersheim, Germany), was cleaned with alcohol and opened with a rotary saw (Dremel 400 Digital, Robert Bosch GmbH, Gerlingen, Germany) at the blunt end (Ø 40 mm). The recipient eggshell was emptied and protected from desiccation by positioning it on a wet and sterile tissue. After 72 hours of incubation, fertilized eggs were opened and placed in a cling film covering a flat dish. The cling film was used to transfer the embryo into the recipient eggshell and removed afterwards. To prevent contamination 1 ml of penicillin (10000 IU/ml) / streptomycin (10000 μg/ml) (Life Technologies Corp., Carlsbad, CA, USA) was added to the egg preventing direct contact to the embryo. The upper part of the outer shell was coated with liquid egg white to fix two layers of cling film and seal the shell. Recipient eggshell cultures were incubated in the same way as the fertilized eggs. On day 19 they got two holes in the upper end of the eggshell for better ventilation. The cling film was lifted on day 20 to make it easier for the chicken to hatch.
Incubation system
A custom made incubator (Key Science Engineering, Blies- Ebersing) has been used as a basis for integration and evaluation of additional technical components. It was equipped with a stand-alone SPS control unit (LOGO! 12/24 RCE, Siemens, München, Germany) with additional I/O blocks (LOGO! DM16/24R, Siemens, München, Germany) for sensors enabling to set and monitor the temperature and humidity in the incubator. A two axis mechanical system was included allowing a scheduled +/- 30º tilt and 360° rotation of the egg with variable speed. Oxygen concentration was measured using an oxygen sensor (GOX 100, Greisinger electronic GmbH, Regenstauf, Germany). Temperature and relative humidity were additionally measured with a datalogger (MSR 160, MSR Electronics GmbH, Seuzach, Switzerland).
Construction and software tools
The incubator and its modifications were completely designed with 3D computer aided design software (Solidworks, Dassault Systens, Velizy Villacoublay, France). The chassis and most of the other parts have been fabricated using rapid prototyping technology with laser sintering (Objet Connex, Stratasys, Rheinmünster, Germany). The sensor and documentation software is written in C# (Visual Studio, Microsoft, Redmont, USA).
Sensors, imaging and electronics
A compact integrated camera module (μEye XS, IDS Imaging Development Systems, Obersulm, Germany) was used for the documentation unit. It is built around an Aptina CMOS with 2592 x 1944 pixels resolution. Platinum based resistivity temperature sensors (pt100, Farnell, Aschheim, Germany) have been integrated at different spots for the measuring of temperature distribution inside the incubator. An open source microcontroller electronics board (Arduino 2009, Arduino) has been used to control all additional electronics and to provide an USB interface to the host PC.
Results
The process of development and evaluation was divided into three parts. First, a lid system was developed and evaluated as it is the core component of the incubation system. In a second step the incubator was designed considering the properties of the lids. The biological evaluation of the incubator with the lids was the final step in our research.
Development and evaluation of a lid system
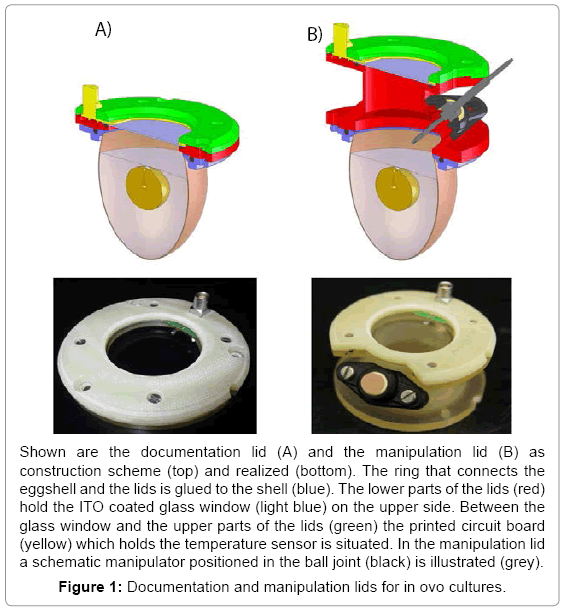
Based on the recipient eggshell culture two different lids have been developed and set up, one for experiments focusing mainly on documentation and one for experiments requiring documentation and manipulation. The lid for documentation (7 cm x 1 cm) has a compact shape and holds a wide glass window allowing insight to the whole scene (Figure 1A). The manipulation lid (7 cm x 6 cm) has an intermediate tube with a ball joint to insert a manipulator in a manner that it can reach the region of interest in the egg (Figure 1B). Both lids feature structural elements holding a large glass window (Ø 38 mm), a pt100 temperature sensor and the printed circuit board (Figure 1). A ring glued to the eggshell forms the mechanical interface to the lids. In order to exclude biocompatibility side effects we tested the influence of four different glues (Histoacryl® B. Braun Melsungen AG, Melsungen, Germany, EPOTEK 301® Epoxy Technology Inc, Billerica, MA, USA, Loctide 9480 A&B Hysol® and Loctide 3090® Henkel AG, Garching, Germany) on the embryonic development. The glues were plotted on the eggshell of fertilized eggs (n=5). Those fertilized eggs were brought to hatch and the hatchlings were checked for malfunctions. Unhatched embryos were freed from their shell and examined. Histoacryl tissue adhesive was the only glue found as not harmful for the embryonic development (data not shown) and additionally had the best handling properties.
Two methods of preventing condensation and keeping the glass of the lids clear were implemented: a heat gun and a resistive heating using an optical transparent indium tin oxide (ITO) coated glass. Both methods are regulated by a feedback control device consisting of a pt100 temperature sensor on the glass, a microcontroller and customized software. The temperature of both anti-condensation methods was found stable over the whole investigated period (day 3-18). The temperature of the heat gun varied up to 0,2°C around the set temperature and the ITO up to 0,1ºC. Both anti-condensation methods were used to determine the minimum temperature necessary to prevent condensation on the glass window. Therefore, embryo cultures were incubated and the temperature of the lid set on distinct values (35,0 to 38,0ºC in 0,5ºC steps). Every 15min the lids were checked for accruing condensation. For both heating methods the lowest temperature without condensation was found to be 36,5°C which was used in further experiments. To investigate potential negative influences of the heated lids hatching experiments were performed. Eggs were closed with both, the manipulation and documentation lid at day 3 and evaluated on hatching healthy chicken. The ITO heating as well as the fan heater managed to keep the window clear over the complete development (day 3-21). All four combinations of heating methods and lid forms led to a repeated hatching of healthy chicken on day 21 (n=2). These chickens were not distinguishable from control animals hatched from fertilized eggs and showed a normal behavior and growth.
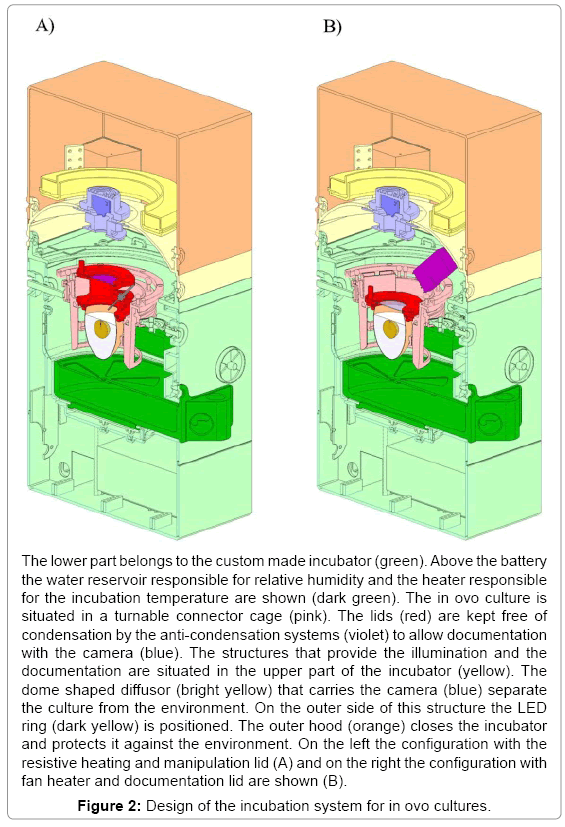
Construction and technical evaluation of an incubator
The incubator was constructed following a modular design (Figure 2). The lower compartment houses the electrical connection, a rechargeable battery, control electronics and heating elements. A logging module facilitates storing of sensor data for more than three weeks. Electronics is separated from the actual incubation section with adjustable vents and sensors for temperature, humidity and oxygen concentration by a removable water reservoir for humidification.
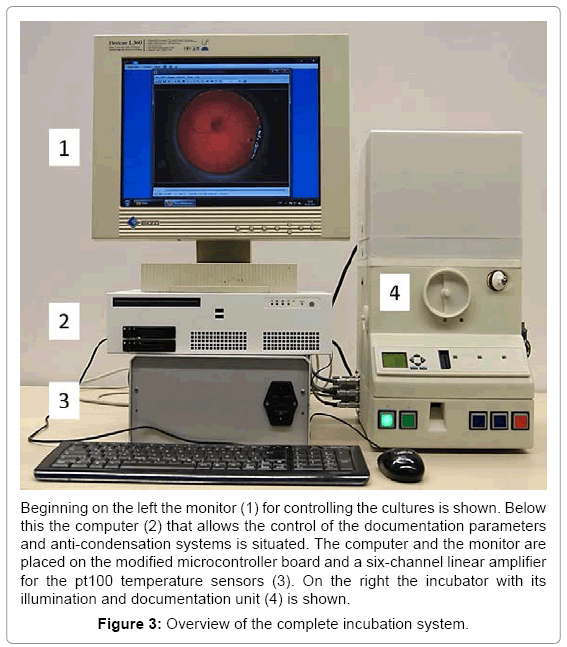
Separated by a thin foil, acting as a vapor barrier, the upper compartment holds the optical documentation system with the camera and the primary light source. This LED based coaxial lighting is implemented for direct and indirect illumination using a semitransparent white dome. The camera with integrated optics is placed coaxial to the rotation axis of the incubator at a working distance of 54 mm. This system has been tested successfully for stability over several periods of three weeks. With a weight of 5 kg and a size of 30 x 50 x 60 cm a very compact system for one egg was obtained (Figure 2). The water reservoir contains up to 600 ml for the regulation of the humidity and supplies the incubator with water for 5 days at 65% relative humidity. A further advantage is the mobility achieved with such a system. The battery for power supply is laid out to bridge 5 hours (temperature, relative humidity and turning) without being connected to an external power supply. With the electronics, the incubator and the computer the complete setup fits easily on a desktop (Figure 3). To check the climatic conditions of the incubation system the parameters were investigated by a long term measurement of temperature set on 37,5ºC and relative humidity set on 65% for 21 days. Both were constant over the measured period (37,5 +/- 1,5ºC and 65 +/- 3%). To prevent hypoxia of the embryo the O2 concentration in the closed system (vents closed) with an embryo over the complete developmental period (day 3-21) was measured. The oxygen-concentration measured did not change significantly over time and ranged about 21%.
Biological evaluation of the incubator
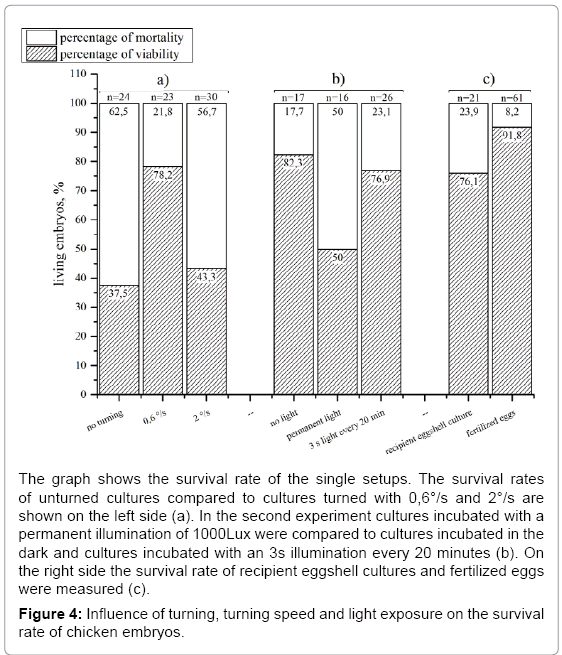
Investigating the survival rate of recipient eggshell cultures we found 76,1% (n=21) of the embryos alive on day 18 compared to 91,8% (n=61) of the control (Figure 4c). The turning of the eggs for documentation is a disadvantage because it changes the position which results in reflections and shadows in the picture. Evaluating the influence of the turning on the survival rate we formed three groups of recipient eggshell cultures: one with turning every 2 hours and a turning speed of 0,6 °/s and one without (n=2) (Figure 4a). The unturned cultures showed a dramatically decrease in viable embryos at day 18 since only half as much embryos as in the turned group survived. The turning speed of the standard incubator (0,6°/s) was then compared to an increased turning speed of 2°/s (Figure 4a). The faster turned eggs showed a strong decrease (to 43,3 % in embryo survival at day 18 (n=2).
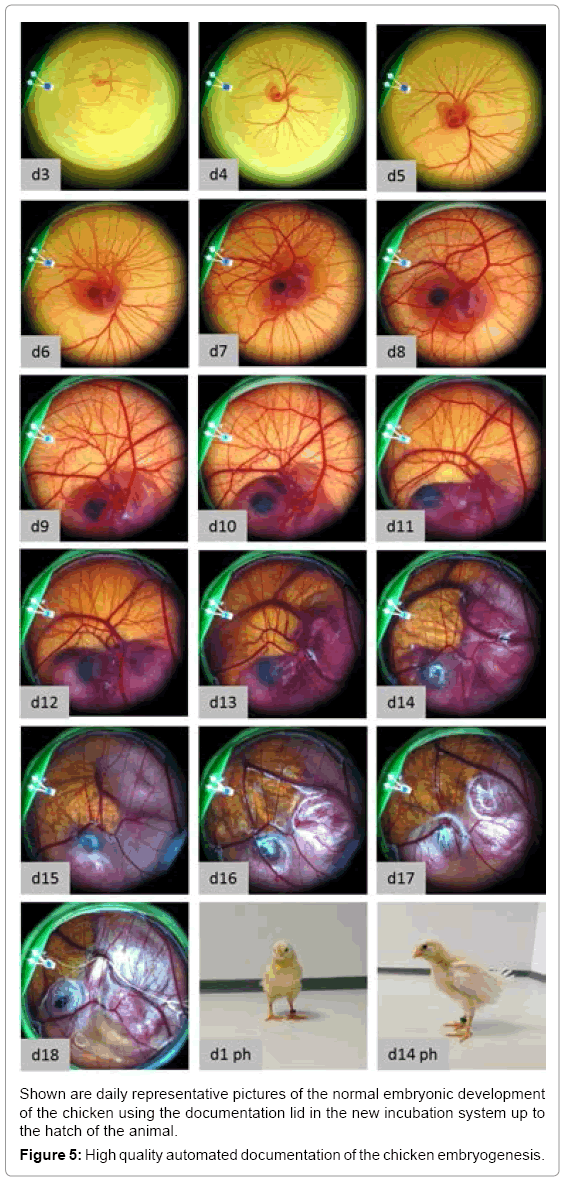
In the unmodified egg the embryo is protected from light during its development. In both the recipient eggshell and the lid system the embryo is exposed to light during manipulation and documentation. Knowing that the eye of the embryo is developed very early (day 3) we investigated if light exposure over a longer period (day 3-18) is influencing the embryo’s survival rate. Therefore, a testing group was continuously exposed with a light of 1100 lux (white LEDs) from day 3 to 18 (Figure 4b) whereas a control group was not exposed to light. The experiments showed a negative influence of permanent lighting on embryo survival at day18. Complete illumination caused a 32% lower survival rate compared to the group without illumination. Thus, we implemented a user programmable illumination and documentation rhythm. In a further experiment the influence of an exposition to light for 3 seconds every 20 minutes on the embryonic survival was tested. This experiment was performed with the same boundary conditions as the previous described experiment with continuous illumination. This periodically illumination did not show a negative influence on the survival of the chicken as 76,9 % of living embryos were found on day 18. Using the whole scale of function of the incubator (temperature, relative humidity, turning, lid heating and time lapse documentation) it was successfully evaluated by hatching experiments. With an automated time-lapse documentation every 20 minutes healthy chicken (Figure 5) hatched and grew to the adult state (n=2) using a modified protocol where the eggs were turned every 20 minutes to avoid pictures of the turned egg to be taken. Keeping one of the hatched chicken, which was female together with three control animals (2 female, one male) it showed good growth and a normal behavior. 136 days after hatching it started to lay eggs as the control animals did too. To negotiate damage on the capability of reproduction we examined the laid eggs. After 3 days of incubation 90% (n=10) of the laid eggs showed well-formed embryos.
Discussion
This study presents the design and proof-of-principle of a new fully automated semi-artificial incubation, manipulation and documentation system for the avian embryogenesis. The system has been evaluated by hatching healthy chicken and documenting their development automatically in high quality. A key requirement therefore is achieved by preventing condensation on the documentation window of the optimized in ovo cultures. This problem appears to our knowledge in all culture systems of the avian embryo and was solved here by the use of controlled heating systems for the lids. Another important feature is the capability of manipulation which is realized in one of the two new lids. With a broad range of manipulators as syringes or pipettes we are able to manipulate the culture without exposing it to the environment and in parallel document the process. This new feature opens a lot of experimental possibilities. Using different kinds of manipulators it is possible not only to insert fluids into the egg but also solids or biological samples like cells or tissues.
The major challenge with the lids was not the construction but the attachment of structural components with a sufficient stability. This excluded the albumen glue used by Perry [14] who did not have enough strength to connect the eggshell and the ring. From the tested glues only the Histoacryl® was found not toxic and with a satisfying stability. We were able to hatch healthy chicken with all configurations of lid and anti-condensation system which indicates our modifications not to interfere with the process of development. This is quite amazing because we noticed the embryogenesis to react sensitive even to smooth manipulations made in previous experiments.
Knowing that the absence of turning [18,19] and a permanent illumination [20] leads to a reduced survival rate of the embryos, we found a setting with an optimal culture survival and a long enough period for time-lapse documentation. The illumination every 20 minutes for 3 seconds (1000lux white LED ring) and the turning of the culture every 20 minutes for 30º allows us to take up to 72 pictures over one day which is more than enough for a quasi-continuous documentation.
The developed incubation system is a multipurpose tool for experimental setups with the chicken embryo, not only to gain further information about the embryonic development but also for other applications using the chicken embryo like the HET-CAM. The HETCAM can easily be performed with the novel incubation system by inserting the test samples with a syringe through the manipulation lid. Another very interesting application for our system could be the use as a test system for toxicity, e.g., embryotoxicity or nanotoxicity. With the increasing use of nanoparticles in e.g. cosmetics and the risks for human and environmental health that is not well researched, [21] testing the exposure of nanoparticles in the living embryo could be an effective way to gain further information. A use of the system for the cultivation of tumor tissue on the CAM was successful done (data not shown) for 8 days and allows researchers the close documentation and manipulation of this in-ovo tumor model.
Additionally, we suggest the system as a calibration and reference tool for the development and establishment of cell-based toxicity tests [22]. This would be facilitated in our system by the use of manipulators with a microfluidic system for the application of toxins on different steps of the development.
An adaption of the system to other avian species as the quail or the duck is given by the adjustable incubation parameters. The control program is capable to perform complex turning routines or cooling steps as required for the breed of waterfowl. With the high flexibility of the parameters the system should also be able to breed reptile eggs as, e.g., tortoises which are incubated with lower temperatures, higher relative humidity and no turning compared to the chicken eggs.
Considering the avian egg as a perfect natural bioreactor for tissue engineering, the incubation system is very advantageous for all types of cell or tissue culture. The embryonic tissue as a proliferative and biological active substrate is an excellent test system to investigate the growth of cells in a biomimetic environment. Application of the biological material is easily possible by using the manipulation lid and a suitable manipulator. Further features like controlled atmosphere with different compositions of gas, e.g., hypoxia and hyperoxia could be realized by small adjustments of the system. A more substantial but straight forward upgrade is the integration of microscopic and fluorescence microscopic documentation. With the integration of filters and a suitable light source, working with labeled cells like in conventional cell and tissue culture is easily possible. Considering these aspects, an incubator for 24 parallel in ovo cultures and equipped with a fluorescence microscope is currently under evaluation.
Acknowledgements
We thank L Peter and M Benecke from IBMT, St. Ingbert for the software implementation and mechanical construction as well as T. Donner from Key Science Engineering, Blies- Ebersing for the construction of the custom made incubator. We further want to acknowledge Adrian Sherman and Helen Sang from Roslin Institute, Edinburgh, Scotland, UK for sharing their expertise in the recipient eggshell culture. This work was supported by the German Bundesministerium für Bildung und Forschung (Förderkennzeichen 1315921A).
References
- Bäumer - Schleinkofer Ä (1993) Die Geschichte der Beobachtenden Embryologie: Die Hühnchenentwicklungals Studienobjektüberzwei Jahrtausende. Verlag Peter Lang. Frankfurt.
- Wolpert L (2004) Much more from the chicken's egg than breakfast--a wonderful model system. MechDev 121: 1015-1017.
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88: 49-92.
- Auerbach R, Kubai L, Sidky Y (1976) Angiogenesis induction by tumors, embryonic tissues, and lymphocytes. Cancer Res 36: 3435-3440.
- Brooks PC, Montgomery AM, Cheresh DA (1999) Use of the 10-day-old chick embryo model for studying angiogenesis. Methods MolBiol 129: 257-269.
- Deryugina EI, Quigley JP (2008) Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol 130: 1119-1130.
- Kain KH, Miller JW, Jones-Paris CR, Thomason RT, Lewis JD, et al. (2014) The chick embryo as an expanding experimental model for cancer and cardiovascular research. DevDyn 243: 216-228.
- Luepke NP (1985) Hen's egg chorioallantoic membrane test for irritation potential. Food ChemToxicol 23: 287-291.
- Schrage A, Gamer AO, van Ravenzwaay B, Landsiedel R (2010) Experience with the HET-CAM method in the routine testing of a broad variety of chemicals and formulations. Altern Lab Anim 38: 39-52.
- Kishore AS, Surekha PA, Sekhar PV, Srinivas A, Murthy PB (2008) Hen egg chorioallantoic membrane bioassay: An in vitro alternative to draize eye irritation test for pesticide screening. Int J Toxicol 27: 449-453.
- Lillico SG, McGrew MJ, Sherman A, Sang HM (2005) Transgenic chickens as bioreactors for protein-based drugs. Drug Discov Today 10: 191-196.
- Elliott JH, Bennett J (1971) Growth of chick embryos in polyethylene bags. PoultSci 50: 974-975.
- Auerbach R, Kubai L, Knighton D, Folkman J (1974) A simple procedure for the long-term cultivation of chicken embryos. DevBiol 41: 391-394.
- Perry MM (1988) A complete culture system for the chick embryo. Nature 331: 70-72.
- Dohle DS, Pasa SD, Gustmann S, Laub M, Wissler JH, et al. (2009) Chick ex ovo culture and ex ovo CAM assay: How it really works. J Vis Exp .
- Isachenko V, Orth I, Isachenko E, Mallmann P, Peters D, et al. (2013) Viability of human ovarian tissue confirmed 5 years after freezing with spontaneous ice-formation by autografting and chorio-allantoic membrane culture. Cryobiology 66: 233-238.
- McGlone (2010) Guide for the Care and use of Agricultural Animals in Research and Teaching. 3rdEdn, Association Headquarters, Champaign, IL, USA.
- Robertson I (1961)The influence of turning on the hatchability of hen’s eggs I. The effect of rate of turning on hatchability. J AgricSci 57: 49-56.
- Lundy H (1969) A review of the effects of temperature, humidity, turning and gaseous environment in the incubator on hatchability of hen’s eggs. In the fertility and hatchability of the hen’s egg. Edinburgh, UK 143-176.
- Tamimie H, Fox M (1967) Effect of continuous and intermittent light exposure on the embryonic development of chicken eggs. CompBiochemPhysiol 20: 793-799.
- Hoet PH, Brüske-Hohlfeld I, Salata OV (2004) Nanoparticles - known and unknown health risks. J Nanobiotechnology 2: 12.
- Seiler AE, Spielmann H (2011) The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat Protoc 6: 961-978.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 18442
- [From(publication date):
September-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 13498
- PDF downloads : 4944