Research Article Open Access
A Novel Electrophoretic Deposition Device: Effects of Alginate Viscosity Grade on Deposition Kinetics
Chris R Jackson, Pavan MV Raja and Satya Prakash*Biomedical Technology and Cell Therapy Research Laboratory, Departments of Biomedical Engineering, Physiology, and Artificial Cells and Organs Research Center, Faculty of Medicine, McGill University, Duff Medical Building, 3775 University Street, Montreal, QC H3A 2B4, Canada
- Corresponding Author:
- Satya Prakash
Biomedical Technology and Cell Therapy Research Laboratory
Departments of Biomedical Engineering, Physiology
and Artificial Cells and Organs Research Center
Faculty of Medicine, McGill University, Duff Medical Building
3775 University Street, Montreal, QC H3A 2B4, Canada
Tel: 514-398-2736
Fax: 514-398-7461
E-mail: satya.prakash@mcgill.ca
Received date: February 02, 2012; Accepted date: February 27, 2012; Published date: February 29, 2012
Citation: Jackson CR, Raja PMV, Prakash S (2012) A Novel Electrophoretic Deposition Device: Effects of Alginate Viscosity Grade on Deposition Kinetics. J Biotechnol Biomaterial S6:002. doi:10.4172/2155-952X.S6-002
Copyright: © 2012 Jackson CR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
A novel device was designed to perform electrophoretic deposition under tightly controlled conditions. The device physical parameters were investigated by depositing three different viscosity grades of sodium alginate hydrogels. A thin metallic rectangular substrate was used to obtain the various time dependant deposition rates of the gels. The resulting deposition curves showed the effective electrophoretic mobilities of the low, medium and high viscosity grade gels were 0.0610 cm2/Vs, 0.0584 cm2/Vs and 0.0909 cm2/V sand that the ratios of gel deposit to solution resistivities were 21.0, 16.2 and 47.5 respectively. Following electrophoretic deposition, the gels were cross-linked in a 0.1 M CaCl2 solution in order to further solidify the gels. Cross-linking reduced the masses of the gels to 50.9 ± 1.8%, 26.7 ± 2.0%, and 28.5 ± 1.3% of their initial masses respectively. Lyophilization was applied to the gels to determine the alginate content of the gels. Immediately after deposition the alginate mass fractions of the low, medium and high viscosity grade gels were 2.92 ± 0.49%, 2.70 ± 0.08% and 2.94 ± 0.15% respectively. Cross-linking caused the mass fraction of alginate to increase significantly to 5.59 ± 0.07%, 7.11 ± 0.37% and 7.02 ± 0.24% respectively. The device in this study provided sufficient data to model the electrophoretic deposition rates. The technique can be expanded to other hydrogel species which can be used in a variety of biomedical and biotechnological applications.
Keywords
Alginate; Electrophoretic deposition; Modelling; Hydrogel; Tissue Engineering; Encapsulation; Rectangular Substrate
Introduction
Electrophoretic deposition (EPD) is a relatively inexpensive method of forming uniform layers or bi-layers on substrates with complex geometries. It has gained much attention in the last 20 years in the development of advanced materials including functional and structural ceramic coatings [1], laminated ceramics [1], biomaterials [2,3], composites [3-6], porous materials, thin films, and nano structured materials [6].
[2,3], composites [3-6], porous materials, thin films, and nano structured materials [6]. In this method, particles are suspended or ions are dissolved in solution before they are driven towards a substrate through the application of an electric field. The particles/ions then form a solid film on one of the electrodes through a number of possible mechanisms including flocculation through accumulation [7], particle charge neutralization [8], particle coagulation [6], and through electrical double layer thinning [9]. EPD has been used on a number of biomaterials such as bioceramics, bioactive materials, nanoparticles, and hydrogels [2]. Porous materials, composites, and textured layers have all been produced for biomedical engineering purposes using this technique. Sodium alginate is a polysaccharide copolymer made of (1-4)-linked β-D mannuronate and C-5 epimer α-L-guluronate blocks. To date, our laboratory has focused on the therapeutic applications of encapsulated alginate systems. The hydrogelis derived from brown algae and has many uses in a variety of fields including tissue engineering. Alginate can be used for both in-situ gelation and immobilization-through-microencapsulation to create injectable scaffolds [10].Such methods have already been used for bone tissue repair [11], cartilage repair [12], skin repair [13] and in neural tissue repair [14]. Recently, alginate has been used to prevent adverse tissue remodeling in damaged myocardial tissue [15]. Alginates are also used for drug delivery [16], cell delivery, enzyme encapsulation and wound dressing [17].This class of materials is widely used for its non-toxicity, biodegradability, and excellent biocompatibility [16].
There are several alginate viscosity grades available which, along with the G/M block ratio, the concentration of cations and the cation species employed during the cross-linking process, greatly affect the gel mechanical and swelling properties [16,18]. The weight average molecular weight has been correlated with viscosity [19] which was shown to have a large impact on the drug release rate of the gel under neutral pH [20,21].The erosion of the gel has been shown to vary depending on the acidity of the environment [22,23]. Previous research has also focused on the importance of cross linking with either Ca2+ or Ba2+ ions in order to improve the mechanical stability of alginate gels [24].
The negatively charged alginate particles are amenable to EPD which results in the formation of a thin uniform alginate-gel film on the desired substrate [7,13,25,26]. Previous studies have focused on the EPD of sodium alginate for biosensor, tissue engineering and corrosion resistance applications [17,26-28]. Sodium alginate undergoes an anodic deposition, and the mechanism has been previously described in literature [25].
Although the feasibility of alginate deposition and co-deposition has been well established, more research is needed to better understand the characteristics of the deposition itself. To the best knowledge of the authors, no study has yet been published that investigated the electrophoretic mobility and the resistivity ratio between the deposited gel and the solution, both of which are required to model the deposition rates. This would allow for a more precise control over the gel thickness which is important for industrial applications. Moreover, the impact of alginate viscosity on EDP has not yet been addressed even though it greatly affects properties such as drug release rate, swelling, and mechanical strength of the gel when it is prepared through direct cross-linking alone.
Materials and Methods
Materials
Three viscosity grades of alginates were used for deposition: low viscosity (LV) alginic acid sodium salt (MP Biomedicals, Solon, Ohio, USA) which was rated at 250 cP at 2% aqueous, medium viscosity (MV) alginate (Sigma Aldrich, St. Louis, Montana, USA) which was rated at 3,500 cP at 2% aqueous, and a high viscosity (HV) alginate (Sigma Aldrich, St. Louis, Montana, USA) which was rated at 14,000 cP at 2% aqueous. The alginates were chosen in order to span the viscosity grades that are commonly manufactured. All alginates were derived from the kelp species M. pyrifera.
Method for determining alginate deposition rates
The EPD device was used to investigate the deposition rates of the three different viscosity grades of alginate. After alginate was deposited on the substrate, the film was rinsed in deionized water before it was briefly exposed to compressed lab air to remove droplets from the surface. The mass was measured before and after deposition using a Mettler Toledo AG204 Delta Range scale (Mettler-Toledo, Greifensee, Switzerland).The mass of the substrates and films were recorded at 1, 2, 3, 5 and 10 minutes. Approximately 1.5 L of solution was used to ensure the alginate concentration remained approximately constant. Depositions were carried out in triplicate at room temperature under static conditions.
Method for cross-linking and evaluation of mass
Cross-linked gels were obtained by submerging the post-depositgels in a 0.1 M CaCl2 (ACP, Montreal, Quebec, Canada) solution overnight at 4°C. The gel masses were evaluated before and after crosslinking and samples were obtained in sextuplicate while the gels were still attached to the substrate.
Method for lyophilization and dry gel mass through
After deposition, sample gels were removed from the substrate, weighed, and placed in petri dishes. A Thermo Savant ModulyoD-115 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to freeze-dry the samples over the span of two days after which changes in mass were recorded. Samples were obtained in triplicate.
Method for determining molecular weight through gel permeation chromatography
Gel permeation chromatography (GPC) was used to differentiate the alginate powders by molecular weight. Experiments were performed courtesy of the Laboratoire de Caractérisation des Matériaux Polymèresau Département de chimie at L’Université de Montreal. The weight average molecular weight (Mw), the number average molecular weight (Mn) and the size average molecular weight (Mz) of the three alginate powders were obtained. Samples were dissolved ina 0.1M NaNO3 solution at a concentration of 10 mg/ml, and experiments were carried out at 35°C. A Polysep-5000 GPC setup (Phenomenex, Torrance, California, USA) with an Ultra hydrogel 500 (Waters, Milford, Massachusetts, USA) column was used with Breeze v.3.20 software (Waters, Milford, Massachusetts, USA). The molecular weights were subsequently used to calculate the viscosities of the different alginate grades using the Mark-Houwink-Sakurada equation.
Method for determining contact angles of dried alginate gels
Contact angles were obtained using a VCA Optima (Billerica, Massachusetts, USA) goniometer with 0.25 μL water droplets. Gels were allowed to dry overnight under vacuum before experimentation. A sample size of 12 was used.
Statistical analysis
Statistical analysis was performed using Minitab software (Minitab, Version 15; Minitab Inc, Pennsylvania, USA). Values are expressed as mean ± standard error. Statistical comparisons were carried out by multiple analyses of variance (ANOVA) or by student t-test where appropriate. Randomized independent sampling was assumed and statistical significance was set at p < 0.05. All interaction terms were treated as fixed terms. Sample sizes varied between 3 and 12, as indicated per experiment.
Results
Design of EPD device
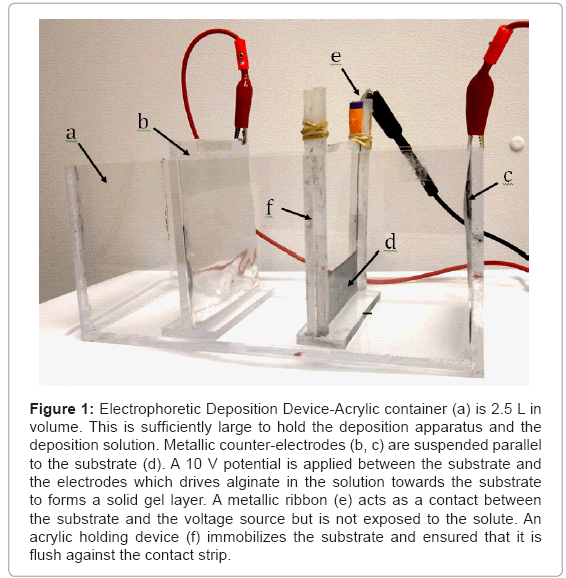
A 10 × 10 × 25 cm box was constructed out of chemically welded acrylic as a means of holding the deposition solution. The 3 × 8 × 0.1 cm rectangular metallic substrate was submerged in the deposition solution and was held in place via a simple holding device that was also constructed out of chemically welded acrylic. To keep the substrate mechanically stable, the 3 cm side of the substrate was pinched between two surfaces whose widths were 0.3 cm. The area exposed to the deposition solution was therefore 43.2 cm2. An electric field was supplied via an Abra AB-3000 (Abra, Champlain, New York, USA) voltage source which remained at a constant voltage of 10.0 V throughout the experiment. Two rectangular counter-electrodes were placed parallel 4 cm from either side of the substrate to obtain a uniform electric field of 2.5 V/cm, which was sufficient to induce alginate deposition. A long thin metallic strip that ran along the side of the substrate holder was used as the contact surface between the voltage source and the substrate. It was positioned such that it was not exposed to the solution. An overview of the device design is shown in Figure 1. In preliminary experiments, it was observed that highly concentrated solutions formed deposits with poor thickness uniformity, so the deposition concentration was kept at 0.4% for all experiments in this paper.
Figure 1: Electrophoretic Deposition Device-Acrylic container (a) is 2.5 L in volume. This is sufficiently large to hold the deposition apparatus and the deposition solution. Metallic counter-electrodes (b, c) are suspended parallel to the substrate (d). A 10 V potential is applied between the substrate and the electrodes which drives alginate in the solution towards the substrate to forms a solid gel layer. A metallic ribbon (e) acts as a contact between the substrate and the voltage source but is not exposed to the solute. An acrylic holding device (f) immobilizes the substrate and ensured that it is flush against the contact strip.
Alginate deposition rates
The EPD device was used to deposit alginate on a flat geometry. The device was designed to be flexible such that other hydrogels can easily be substituted in the deposition solution. Moreover, a codeposition can also be performed with another particle. The device is an excellent tool for modeling the deposition rates as the solution is geometrically constrained to the same cross-section as the substrate, ensuring the electric field is approximately constant over the substrate surface. Moreover, a large volume of deposition solution can be held by the device, allowing for a constant-concentration approximation assumption to be taken, although it must be supported with rigorous calculations based on the mass of the deposit layer formed.
The mass of the alginate films increased approximately linearly for the first few time points, but over time the deposition rates decreased. The surface was smooth for low deposition times, but dimples and variations in colour concentration appeared as deposition time approached 10 minutes.
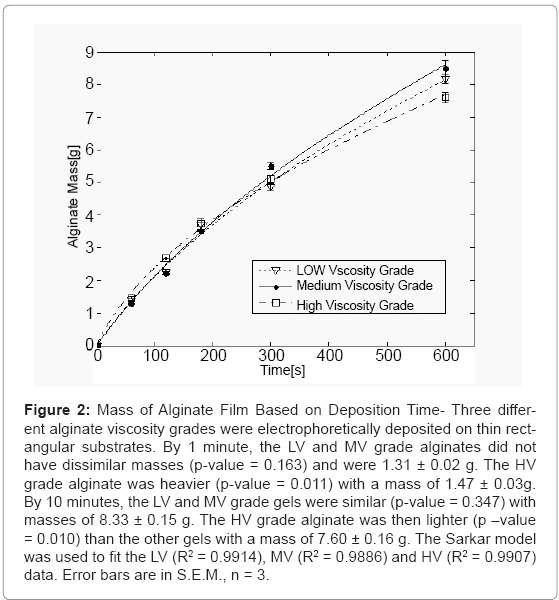
Figure 2 shows the alginate film mass as a function of time. For the first 180 seconds, the LV and MV grade alginates deposited at the same rate which was slightly below that of the HV grade alginate. By 10 minutes, the HV gel had the lowest mass, implying that the HV grade alginate deposition rate was most severely slowed down in the later stage of the deposition. By the end of the deposition, the LV and MV gels had different masses, with the MV being the higher of the two. All three gels were found to be significantly different from one another with p-values <0.005.
Figure 2: Mass of Alginate Film Based on Deposition Time- Three different alginate viscosity grades were electrophoretically deposited on thin rectangular substrates. By 1 minute, the LV and MV grade alginates did not have dissimilar masses (p-value = 0.163) and were 1.31 ± 0.02 g. The HV grade alginate was heavier (p-value = 0.011) with a mass of 1.47 ± 0.03g. By 10 minutes, the LV and MV grade gels were similar (p-value = 0.347) with masses of 8.33 ± 0.15 g. The HV grade alginate was then lighter (p –value = 0.010) than the other gels with a mass of 7.60 ± 0.16 g. The Sarkar model was used to fit the LV (R2 = 0.9914), MV (R2 = 0.9886) and HV (R2 = 0.9907) data. Error bars are in S.E.M., n = 3.
Cross linking mass reduction
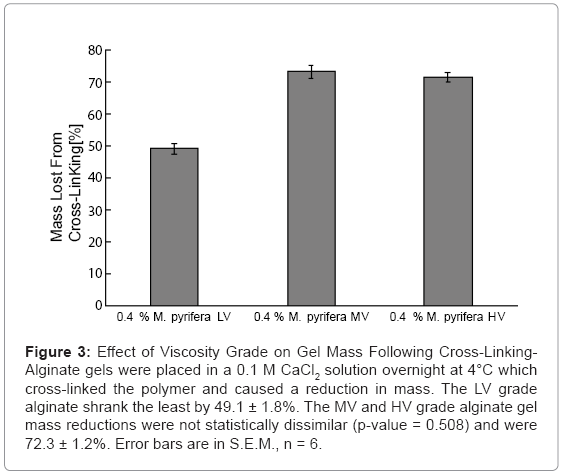
The cross-linked-gel mass was calculated as a percent of the initial mass and is shown in Figure 3. The MV and HV gels had similar changes in mass (p-value = 0.508) with a reduction of 72.3 ± 1.2%. The LV gel mass decreased the most with a reduction of 49.1 ± 1.8%.
Figure 3: Effect of Viscosity Grade on Gel Mass Following Cross-Linking- Alginate gels were placed in a 0.1 M CaCl2 solution overnight at 4°C which cross-linked the polymer and caused a reduction in mass. The LV grade alginate shrank the least by 49.1 ± 1.8%. The MV and HV grade alginate gel mass reductions were not statistically dissimilar (p-value = 0.508) and were 72.3 ± 1.2%. Error bars are in S.E.M., n = 6.
Gel description
All un-cross-linked gels were opaque, with a yellow tint. The side of the gel that was exposed to the deposition solution had bumps, while the substrate side of the gel was flat. The opacity of the un-cross-linked gels decreased with viscosity. The cross-linked gels were more opaque than their un-cross-linked counterparts. Images of gels obtained after 5 minute depositions are shown in Figure 4. A water displacement test was performed on the gels and showed they all had a density of approximately 1.0 g/cm3.
Effect of lyophilization on alginate gels mass
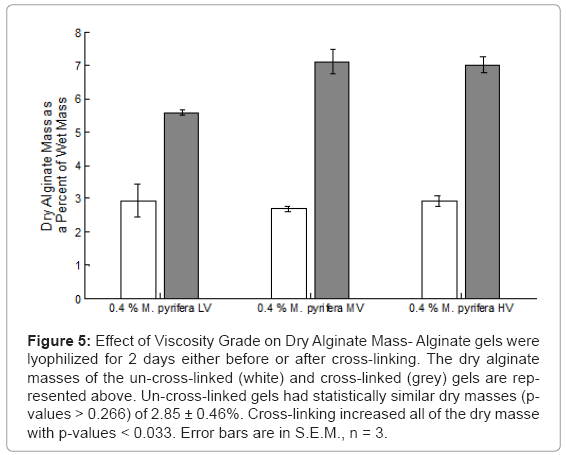
The dry alginate mass was calculated as a percent of the post-deposit gel mass. The dry alginate contents were statistically similar (p-values > 0.266) for all three gels with dry mass fractions of 2.85± 0.46% show in Figure 5. When the gels were cross-linked, their water contents were reduced. The MV and HV gels had similar dry masses (p = 0.839) of 7.07 ± 0.46%, while the LV gel has a dry mass of 5.59 ± 0.07%.
Figure 5: Effect of Viscosity Grade on Dry Alginate Mass- Alginate gels were lyophilized for 2 days either before or after cross-linking. The dry alginate masses of the un-cross-linked (white) and cross-linked (grey) gels are represented above. Un-cross-linked gels had statistically similar dry masses (pvalues > 0.266) of 2.85 ± 0.46%. Cross-linking increased all of the dry masse with p-values < 0.033. Error bars are in S.E.M., n = 3.
Molecular weight through gel permeation chromatography
The values of Mn, Mz and Mw are shown in Table 1. It was shown that for all measurements except Mn, the molecular weight increased with viscosity grade. This indicates that although the majority of the mass of the HV alginate was found in the higher molecular weight range, there was a larger presence of smaller molecules. Increased molecular weights are associated with higher alginate viscosity [29,30], and the relation between the two have been discussed in literature [31,32].
| Alginate Grade | Mn | Mw | Mz | [η] | C' | η2% | η0.4% |
|---|---|---|---|---|---|---|---|
| Units | [kDa] | [kDa] | [kDa] | [ml/g] | [%] | [cP] | [cP] |
| 0.4% M. pyrifera LV | 196 | 643 | 1,652 | 208 | 1.92 | 250 | 24.4 |
| 0.4% M. pyrifera MV | 196 | 779 | 2,055 | 236 | 1.70 | 3500 | 269 |
| 0.4% M. pyrifera HV | 178 | 981 | 2,446 | 275 | 1.46 | 14,000 | 805 |
Table 1: Viscosities of Deposition Solutions- The molecular weights of the three sample alginates were obtained courtesy of the Laboratoire de Caractérisation des Matériaux Polymères au Département de chimie at L’Université de Montreal. From Mw, [η] was calculated using the Mark-Houwink-Sakurada equation [32], which allowed for the calculation of the critical concentration C'. Using the viscosities of the 2% solutions which were provided by the manufacturers, the 0.4% viscosities were then calculated.
Dried Alginate Gels’ Contact Angles
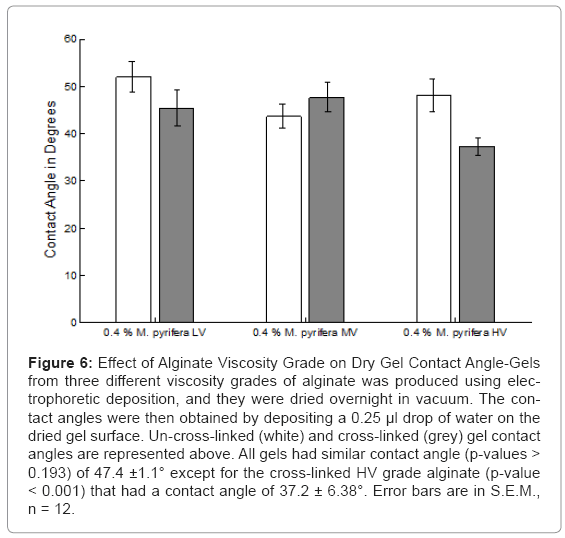
Contact angles are expressed from the water side of the droplet, so angles <90° are hydrophilic. All gels had statistically similar (p-values > 0.193) contact angles of 47.4 ± 1.1° except for the cross-linked HV gel whose contact angle was 37.2 ± 6.38° as shown in Figure 6. As the dried gel is both highly hydrophilic and porous, droplets would absorb quickly, which warped the surface of the dried gels. Contact angles were therefore taken quickly after droplets were deposited before warping occurred.
Figure 6: Effect of Alginate Viscosity Grade on Dry Gel Contact Angle-Gels from three different viscosity grades of alginate was produced using electrophoretic deposition, and they were dried overnight in vacuum. The contact angles were then obtained by depositing a 0.25 μl drop of water on the dried gel surface. Un-cross-linked (white) and cross-linked (grey) gel contact angles are represented above. All gels had similar contact angle (p-values > 0.193) of 47.4 ±1.1° except for the cross-linked HV grade alginate (p-value < 0.001) that had a contact angle of 37.2 ± 6.38°. Error bars are in S.E.M., n = 12.
Discussion
EPD Device
EPD devices commonly employ large baths of deposition solution while completely neglecting the effects of solution geometry on the local electric field strength. For substances that deposit relatively much quicker than ceramics such as hydrogels, this means that an uneven deposition can become very pronounced, which can skew the results for modeling purposes. Boccaccini et al. for example describe an EPD device in which the substrate was held in an excessively large solution bath [2]. Although such a container is flexible in that unspecific substrate geometries can be used, it is not ideal for modeling the deposition rates. Non-uniform coatings may also have played a role in the non-linear correlation between chitosan concentration and deposition rate [33]. In another study, quartz crystal microbalance method of measuring deposit mass was used to yields significantly more time points [27], but it cannot be used for extensive deposition times where the deposit resistance becomes significant.
The device presented in this study overcomes the above mentioned obstacles. The container shape forces the deposition solution into a geometry such that the electric fields are parallel, ensuring the electric field strength is uniform over the surface of the substrate. The ability to weigh deposits after extended deposition times allowed for the characterization of gel deposition rate well beyond the non-linear range which has not been attempted before for alginate. The device could easily be used with other charged polymers, hydrogels, ceramics and nanoparticles as well as other suspension mediums such as ethanol and other organic solvents as long as the electric field strength and direction is controlled appropriately.
Alginate Deposition Rates
EPD rates were first modeled by Hamaker [34] who obtained the following relation describing the process:
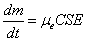
where μe is the electrophoretic mobility [cm2/Vs], C is the concentration [g/cm3], S is the surface area of thesubstrate [cm2], E is the electric field strength [V/cm], t is time [s] and m is the mass of the deposit [g]. Although this relationship was true for short deposition times, the deposition rate slowed down throughout longer deposition times.
To explain this phenomenon, longer depositions are modelled either as constant current or constant voltage conditions. These are further classified into the two scenarios where the deposition solution concentration is or isn’t kept constant. Under constant voltage/constant concentration conditions, the buildup of a resistive layer of deposit slows down the deposition rate with time. Sarkar and Nicholson PS used the Hamaker equation to obtain the relation for deposit mass under these conditions [9]:
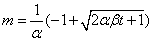
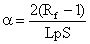
β = μe SCE
Where L is the distance between electrodes [cm], Rf is the ratio of deposit resistivity to the suspension resistivity, and ρ is the density of the deposit [g/cm3].
In many EPD processes including those involving ceramics, the solute is the only species deposited on the substrate. In the case of hydrogels, however, the solvent also forms part of the deposited mass. It is therefore more practical to model the deposition rate in terms of the total mass of the deposited hydrogel and not just the polymer species. The alginate deposition curves obtained from our EPD device were fit to the above Sarkar equation using MatLab R2010a (MathWorks, Natick, Massachusetts, USA) cf tool function and the results are shown in Figure 2. Values for α and β, as well as the calculated values of μe and Rf are shown in Table 2. It was found that μe and Rf for the LV and MV grade alginates were similar, however both values were noticeably higher for the HV gel.
| Alginate Grade | α | β | μe | Rf |
|---|---|---|---|---|
| Units | [1/g] | [g/s] | [cm2/Vs] | [unitless] |
| 0.4% M. pyrifera LV | 0.2302 | 0.02634 | 0.0610 | 21.0 |
| 0.4% M. pyrifera MV | 0.1759 | 0.02524 | 0.0584 | 16.2 |
| 0.4% M. pyrifera HV | 0.5377 | 0.03927 | 0.0909 | 47.5 |
Table 2: Deposition Rate Modelling Parameters: Values of α and β were obtained by curve-fitting the mass vs. time curves shown in Figure 2 with the Sarkar equation for constant-voltage/constant-concentration deposit mass. From this, the electrophoretic mobility (μe) and deposit-solution resistivity ratios (Rf) were obtained.
The first two terms of the Maclaurin series for the above equation yields:
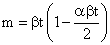
As the Maclaurin series for the Sarkar equation is an alternating series, this equation can be used to show that the error associated with the Hamaker equation is negligible for values of 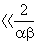
The electrophoretic mobility is an empirical value that results from the balance between the electrostatic forces and the friction forces exerted on the particle. Although the viscosity grade is a good indicator of the friction forces resisting particle motion towards the substrate, the electrostatic force is much more complex, and depends on particle charge, inter-particle interactions, and local pH which vary along the distance between the electrodes [35]. The viscosity of the HV gel indicated that the friction force would have been higher during EPD compared to the other two gels. The HV μe was nevertheless greater, indicating that the increased friction was overcome by an even greater increase in electrostatic force. The HV gel deposition rate slowed down the most throughout the run due its larger Rf .
Cross-linking
The MV and HV gels had a larger mass reduction following cross-linking than did the LV gel. This is because the MV and HV alginate particles were larger, offering more sites for Ca2+ particle bridging. This resulted in a higher degree of cross-linking, and therefore a reduction in swelling properties. In literature, dry alginate and alginate microcapsules were also shown to have changes in mass increase with viscosity grade when they were moved from cross-linking solutions to water [22,36,37].When alginate microcapsules produced through an emulsion technique were placed in water [38], a slightly higher change in mass was observed compared gels produced using EPD subjected to a cross-linking solution.
All gels had approximately the same density so the change in volume was the same as the change in mass. Gel opacity was caused by micro-bubbles formed at the cathode. As bubble transportation from the cathode to the anode increased as viscosity decreased, it follows that opacity also increased as viscosity grade decreased. Gels were qualitatively observed to have improved mechanical properties following cross-linking. This is especially true for the LV grade alginate. Future works should focus on a qualitative assessment of the gel.
Contact angle
The HV gels contact angle following cross-linking was likely smaller due to Ca2+ uptake. All surfaces were shown to be highly hydrophilic, with contact angles below 47.4°C. Hydrophilic materials are ideal for mammalian cell cultures [39] due to an uptake in adhesive proteins [40-42]. As hydrophobic materials are commonly antithrombotic and resistant to fibrin sheath formation [43], the gels may have potential in tissue engineering and implant coatings applications due to their increase biocompatibility. EPD would be especially useful as it would be possible to coat arbitrarily shaped tissue engineering scaffolds, so long as they are conductive. The hydrophilic surface is also ideal for immobilizing hydrophilic proteins.
Viscosity calculations from molecular weights
It was shown that cross-linking reduced the mass for the MV and HV grade alginates to a greater extent than it did for the LV grade alginate. This is in accordance with previous studies that have shown a greater degree of cross-linking in alginates of higher molecular weight [44]; however it may also be due to differences in the fraction of G blocks in the polymer, which also have a higher affinity for Ca2+ ions. The viscosity grade increased with Mw as shown in previous studies [19]. The loss of mass that occurred during cross-linking was least extreme in the LV gels, which resulted in them having the smallest change in water mass fraction after cross-linking out of the three gels.
The viscosities of the solutions used in the experiments were approximated using the relation obtained by Morris et al [31] whereby the concentration dependent viscosity of a random coil polysaccharide varied as C3.3 and C1.4 for dilute and highly concentrated solutions respectively. The transition between dilute and highly concentrated solution occurs at concentration C’:

where [η] is the intrinsic viscosity [ml/g]. Values of [η] were calculated from experimentally obtained values of Mw using the Mark-Houwink- Sakurada relation [31,32]:
where K and a are the Mark-Houwink-Sakurada constants. As the molecular weights for all alginates were above 300 kDa, values of 0.0305 and 0.66 were used for K and a respectively [31,32]. Viscosities of 2% solutions provided by the respective manufacturers were used to calculate the viscosities of the solutions at the critical concentrations and at 0.4%by scaling accordingly. Relevant values are shown in (Table 1). It was thus shown that the viscosities of the 0.4% solutions spanned a total of 1.5 orders of magnitude.
Conclusion
A novel EDP device was designed to effectively deposit alginate on a flat substrate. Alginate films were then produced on thin steel rectangles using three alginates of different viscosity grades. The deposition mass was quantified and was used to show that the process could be modeled using the Sarkar equation for a constant voltage/constant concentration EPD.A change in mass was observed following cross-linking using CaCl2 solution, with the LV grade alginate having the smallest change in mass. The dry alginate content was similar for all samples before crosslinking, but it was lower for the cross-linked LV grade alginate than for the other two. Although the viscosity grade affected the deposition rate, the Sarkar model was still accurate as long as appropriate μe and Rf values were used. Although results indicated that many properties varied with viscosity grade, it is possible that properties may become more extreme with a larger range of grades. These results will be useful in the design and manufacturing of devices that incorporate EPD of sodium alginate. Moreover, the methods outlined in this paper can be extrapolated to model and understand other hydrogel systems.
Acknowledgements
A special thanks is given to Dr. Hani Saleh Fadhl Al-Salami of McGill University for his help with statistical analysis. Pierre Ménard-Tremblay is also to thank for performing the Gel Permeation Chromatography experiment and for running the analysis at l’Université de Montréal. The authors would also like to acknowledge Dr. Jamal Daoud of McGill University for helping out with the lyophilization procedure and the contact angle test.
The authors would also like to acknowledge the Canadian Institute of Health Research (CIHR) grant (MOP 93641) to Dr. S. Prakash, and for funding provided by NSERC.
References
- Van der Biest O, L Vandeperre, Put S, Anne G, Vleugels J (2007) Laminated and functionally graded ceramics by electrophoretic deposition. Adv Sci Technol 45: 1075-1084.
- Boccaccini AR, Keim S, Ma R, Li Y, Zhitomirsky (2010) Electrophoretic deposition of biomaterials. J R Soc Interface 7: S581-S613.
- Bai Y, Kim KA, Park IS, Lee SJ, Bae TS et al. (2011) In situ composite coating of titania-hydroxyapatite on titanium substrate by micro-arc oxidation coupled with electrophoretic deposition processing. Materials Science and Engineering B 176: 1213-1221.
- Cho J, Schaab S, Roether J, Boccaccini A (2008) Nanostructured carbon nanotube/TiO2 composite coatings using electrophoretic deposition (EPD). J Nanopart Res 10: 99-105.
- Sarkar P, Huang X, Nicholson PS (1993) Zirconia / Alumina Functionally Gradiented Composites by Electrophoretic Deposition Techniques. J Am Ceram Soc 76: 1055-1056.
- Corni I, MP Ryan, Boccaccini AR (2008) Electrophoretic deposition: From traditional ceramics to nanotechnology. J Eur Ceram Soc 28: 1353-1367.
- Hamaker HC, Verwey EJW (1940) Part II.-(C) Colloid stability. The role of the forces between the particles in electrodeposition and other phenomena. Trans Faraday Soc 35: 180-185.
- Grillon F, Fayeulle D, Jeandin M (1992) Quantitative Image-Analysis of Electrophoretic Coatings. J Mater Sci Lett 11: 272-275.
- Sarkar P, Nicholson PS (1996) Electrophoretic deposition (EPD): Mechanisms, kinetics, and application to ceramics. J Am Ceram Soc 79: 1987-2002.
- Prabhakaran MP, Venugopal J, Kai D, Ramakrishna S (2011) Biomimetic material strategies for cardiac tissue engineering. Materials Science & Engineering C 31: 503-513.
- Tan R, Feng Q, She Z, Wang M, Jin H, et al. (2010) In vitro and in vivo degradation of an injectable bone repair composite. Polym Degrad Stab 95: 1736-1742.
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, et al. (2007) Microfluidic scaffolds for tissue engineering. Nat Mater 6: 908-915.
- Hunt NC, Shelton RM, Grover L (2009) An alginate hydrogel matrix for the localised delivery of a fibroblast/keratinocyte co-culture. Biotechnol J 4: 730-737.
- Li L, Davidovich AE, Schloss JM, Chippada U, Schloss RR, et al. (2011) Neural lineage differentiation of embryonic stem cells within alginate microbeads. Biomaterials 32: 4489-4497.
- Leor JN Landa, Miller L, Feinberg MS, Holbova R, et al. (2008) Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117: 1388-1396.
- Hernandez RM, Orive G, Murua A, Pedraz JL (2010) Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev 62: 711-730.
- Becker TA, Kipke DR, Brandon T (2001) Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization. J Biomed Mater Res 54: 76-86.
- Martinsen A, Skjakbraek G, Smidsrod O (1989) Alginate as Immobilization Material .1. Correlation between Chemical and Physical-Properties of Alginate Gel Beads. Biotechnol Bioeng 33: 79-89.
- Schneider S, Feilen PJ, Kraus O, Haase T, Sagban TA, et al. (2003) Biocompatibility of Alginates for Grafting: Impact of Alginate Molecular Weight. Artif Cells Blood Substit Biotechnol 31: 383-394.
- Sperger DM, Fu S, Block LH, Munson EJ (2011) Analysis of composition, molecular weight, and water content variations in sodium alginate using solid-state NMR spectroscopy. J Pharm Sci 100: 3441-52.
- Imai T, Kawasaki C, Nishiyama T, Otagiri M (2000) Comparison of the pharmaceutical properties of sustained-release gel beads prepared by alginate having different molecular size with commercial sustained-release tablet. Pharmazie 55: 218-222.
- Efentakis M, Koutlis A (2001) Release of Furosemide from Multiple-Unit and Single-Unit Preparations Containing Different Viscosity Grades of Sodium Alginate. Pharm Dev Technol 6: 91-98.
- Efentakis M, G Buckton (2002) The Effect of Erosion and Swelling on the Dissolution of Theophylline from Low and High Viscosity Sodium Alginate Matrices. Pharm Dev Technol 7: 69-77.
- Zimmermann U, Manz B, Hillgartner M, Zimmermann H, Zimmermann D, et al. (2004) Cross-linking properties of alginate gels determined by using advanced NMR imaging and Cu2+ as contrast agent. Eur Biophys J 33: 50-58.
- Yokoyama F, Fujino T, Kimura K, Yamashita Y, Nagata K, et al. (1998) Formation of optically anisotropic alginic acid gels under DC electric fields. Eur Polym J 34: 229-234.
- Shi XW, Tsao CY, Yang XH, Liu Y, Dykstra P, et al. (2009) Electroaddressing of Cell Populations by Co-Deposition with Calcium Alginate Hydrogels. Adv Func Mater 19: 2074-2080.
- Cheong M, Zhitomirsky I (2008) Electrodeposition of alginic acid and composite films. Colloids Surf A Physicochem Eng Asp 328: 73-78.
- Liu CH, Guo XL, Cui HT, Yuan R (2009) An amperometric biosensor fabricated from electro-co-deposition of sodium alginate and horseradish peroxidase. J Mol Catal B Enzym 60: 151-156.
- Draget KI, G Skjåk Bræk, O Smidsrød (1994) Alginic acid gels: the effect of alginate chemical composition and molecular weight. Carbohydrate Polymers 25: 31-38.
- Mancini F, Montanari L, Peressini D, Fantozzi P(2002) Influence of Alginate Concentration and Molecular Weight on Functional Properties of Mayonnaise. LWT - Food Sci Technol 35: 517-525.
- Morris ER, Cutler AN, Ross-Murphy SB, Rees DA, Price J (1981) Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydrate Polymers 1: 5-21.
- Vold IMN, Kristiansen KA, Christensen BE (2006) A Study of the Chain Stiffness and Extension of Alginates, in Vitro Epimerized Alginates, and Periodate-Oxidized Alginates Using Size-Exclusion Chromatography Combined with Light Scattering and Viscosity Detectors. Biomacromolecules 7: 2136-2146.
- Simchi A, Pishbin F, Boccaccini AR (2009) Electrophoretic deposition of chitosan. Materials Letters 63: 2253-2256.
- Hamaker HC (1940) Formation of a deposit by electrophoresis. Trans Faraday Soc 35: 279-287.
- Besra L, Liu M (2007)A review on fundamentals and applications of electrophoretic deposition (EPD). Prog Mater Sci 52: 1-61.
- Efentakis M, Buckton G (2002) The Effect of Erosion and Swelling on the Dissolution of Theophylline from Low and High Viscosity Sodium Alginate Matrices. Pharm Dev Technol 7: 69-77.
- Polk A, Amsden B, De Yao K, Peng T, Goosen MFA (1994) Controlled release of albumin from chitosan—alginate microcapsules. J Pharm Sci 83: 178-185.
- Acarturk ST (1999) Calcium alginate microparticles for oral administration: I: effect of sodium alginate type on drug release and drug entrapment efficiency. J Microencapsul 16: 275-290.
- Webb K, Hlady V, Tresco PA (1998) Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J Biomed Mater Res 41: 422-430.
- Steele JG, McFarland C, Dalton BA, Johnson G, Evans MDM, et al. (1994) Attachment of human bone cells to tissue culture polystyrene and to unmodified polystyrene: the effect of surface chemistry upon initial cell attachment. J Biomater Sci Polym Ed 5: 245-257.
- Iuliano DJ, Saavedra SS, Truskey GA (1993) Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res 27: 1103-1113.
- Grinnell F, Feld MK (1981) Adsorption characteristics of plasma fibronectin in relationship to biological activity. J Biomed Mater Res 15: 363-381.
- Bae JS, Seo EJ, Kang IK (1999) Synthesis and characterization of heparinized polyurethanes using plasma glow discharge. Biomaterials 20: 529-537.
- Heng PWS, Lee HY, Chan LW, Dolzhenko AV (2006) Influence of viscosity and uronic acid composition of alginates on the properties of alginate films and microspheres produced by emulsification. J Microencapsul 23: 912-927.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16934
- [From(publication date):
specialissue-2014 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12288
- PDF downloads : 4646