A Novel Development for Dye Sensitized Solar Cells Solid-state Electrolytes without the Use of a Clean Room
Received: 19-Sep-2016 / Accepted Date: 05-Oct-2016 / Published Date: 10-Oct-2016
Abstract
Dye sensitized solar cells (DSSCs) solid-state electrolytes were prepared by mixtures method and applied to dye sensitized solar cells (DSSCs). The cells were coated a conducting glass sheet by means of doctor blade technique. The solid state electrolytes were prepared by adding KI and I2 into solid state electrolytes composed of 1,2,4,5- tetrakisbromomethyl benzene(TB), γ-butyrolactone (GBL), propylene carbonate(PC), 1-methyl-3-propylimidazodium iodide (MPI), poly (styrene-co-acrylonitrile)(P(S-A)) and ethylene carbonate (EC). The solid state electrolytes mixtures became homogenous and could not flow under sintering at temperature of 70-80°C gelated with sample 1, 2, 3 and 4. The dye sensitized solar cells (DSSCs) based on the solid state electrolytes yielded an overall light to electrical energy conversion efficiency of 2.49%, 2.56%, 2.16% and 2.18% respectively, under irradiation of 80 mW/cm2.
Keywords: Dye sensitized solar cells (DSSCs); Solid-state electrolytes; Energy conversion efficiency; Iodine
19055Introduction
During the last quarter of the 20th century to the 21st century, many researchers have shown an increase of interest in the development of renewable and non-conventional energy sources having appreciable energy conversion characteristics without pollution. The use of alternative energy sources that generate electricity in an intermittent basis, for example, solar energy and wind power, requires low cost and high efficiency electricity storage systems. Dye sensitized solar cells (DSSCs) have generated considerable research interest for the past decade [1], because of their high-energy conversion efficiency (~10%) and low production cost [2,3].
Recently, some researches have been reported on the high performance dye sensitized solar cells (DSSCs) employing quasi-solid polymer electrolytes [4] prepared polymer electrolytes consisting of poly (epichlorohydrin-co-ethylene oxide) (Epychlomer), NaI and I2 and applied to solid-state dye sensitized solar cells (DSSCs). Epychlomer electrolytes show relatively high ionic conductivity of 1.5×10-5 S/cm at 30°C and solar cells efficiency of 2.6% at 100 mW/cm2 due to the reduced crystallinity of polymer chains. Stergiopoulos et al. also reported highly efficient nanocrystalline titanium dioxide (TiO2) solar cells employing binary poly (ethylene oxide) (PEO)/titania/LiI/I2 electrolyte. Their photoelectrochemical cells resulted in high overall energy conversion efficiency, i.e., 4.2% at 65.6 mW/cm2. Very recently, Stergiopoulos reported that a supramolecular electrolyte was designed for use in dye sensitized solar cells (DSSCs) by modifying low molecular weight polyethylene glycol (Mw=1000 g/mol) at both chain ends with functional groups having quadruple hydrogen bonding sites [5]. The overall conversion efficiency of these dye sensitized solar cells (DSSCs) was 3.34% at 100 mW/cm2 (4.59% at 42.9 mW/cm2). They also have demonstrated that composite polymer electrolytes consisting of PEO, fumed nanosized silica, an iodide salt, and iodine to provide improved dye sensitized solar cells (DSSCs) performance up to 4.5% at 100 mW/cm2, which is one of the highest value sever reported for dye sensitized solar cells (DSSCs) employing solid polymer electrolytes [1].
In this paper, we report the method of preparation of solid-state electrolytes applied and use them for dye sensitized solar cells (DSSCs) device. We have fabricated solar cell using nanocrystalline titanium dioxide (TiO2) thin films with sol-gel method based on doctor blade technique. The solid state electrolytes were prepared by adding KI in I (10 g/1 g) into solid state electrolytes consisting 1,2,4,5–tetrakisbromo methyl benzene (TB), γ-butyrolactone (GBL), propylene carbonate (PC), 1-methyl-3-propyl imidazodium iodide (MPI), poly (styrene– co–acrylonitrile) (P(S-A)) and ethylene carbonate (EC). The dye sensitized solar cells (DSSCs) were tested yielded an overall light to electrical energy conversion efficiency (%), under irradiation of 80 mW/cm2.
Experimental
Preparation nanocrystalline TiO2
Nanocrystalline titanium dioxide (TiO2) films were made by following procedure. The sol-gel processing of titanium dioxide (TiO2) was prepared using by mixing powder 79.90%, mixed into 0.1 M nitric acid aqueous solution in an iso-propanol solution, and distilled water was added for adjusted the pH value, pH 3-4. A conducting glass sheet transparent conducting oxide glass was coated with titanium dioxide (TiO2) film with doctor blade technique. Finally, the titanium dioxide (TiO2) porous film was sintered by firing the conducting glass sheet at 450°C for 30 min. After that, a titanium dioxide (TiO2) porous film electrode was immersed to absorb in the sensitizing dye bis (tetrabutylammonium)-cis-di(thiocyanato)-N,N-bis (4-carboxylato-4- carboxylic acid-2,2-bipyridine) ruthenium(II) (or N719 dye) for 18-24 h, adequately and then the other impurities were washed up with anhydrous ethanol and dried in moisture-free air.
Solid state electrolytes
A series of mixtures were prepared by adding 0.5 g potassium iodide (KI), 0.05 g iodine (I2), into a consisting sample 1, 1.0 mL propylene carbonate (PC) 0.1 g 1,2,4,5–tetrakisbromomethyl benzene (TB) 1.5 g ethylene carbonate (EC) 1.0 mL 1–methyl–3–propylimidazolium iodide (MPI) and 1.5 g poly(styrene–co–acrylonitrile) (P(S-A)), sample 2, 1.0 mL propylene carbonate (PC), 0.1 g 1,2,4,5– tetrakisbromomethyl benzene 2 mL γ–butyrolactone (GBL) 1.0 mL 1– methyl–3–propylimidazolium iodide (MPI) and 1.5 g poly (styrene– co–acrylonitrile) (P(S-A)), sample 3, 1.5 g ethylene carbonate (EC) 0.1 g 1,2,4,5–tetrakisbromomethyl benzene (TB) 2 mL γ–butyrolactone (GBL) 1.0 mL 1–methyl–3–propylimidazolium (MPI) and 1.5 g poly (styrene–co–acrylonitrile) (P(S-A)), sample 4, 1.0 mL propylene carbonate (PC) 0.1 g 1,2,4,5–tetrakisbromomethyl benzene (TB) 1.5 g ethylene carbonate (EC) 2 mL γ–butyrolactone (GBL) 1.0 mL 1– methyl–3–propylimidazolium iodide (MPI) and 1.5 g poly(styrene– co–acrylonitrile) (P(S-A)). The solid-state electrolytes were obtained when the mixtures became homogenous and could not flow under stirring at temperature of 70-80°C.
Results And Discussion
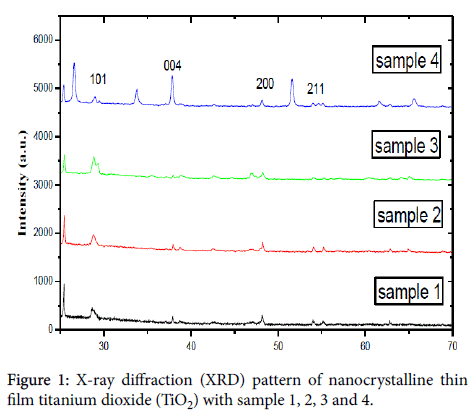
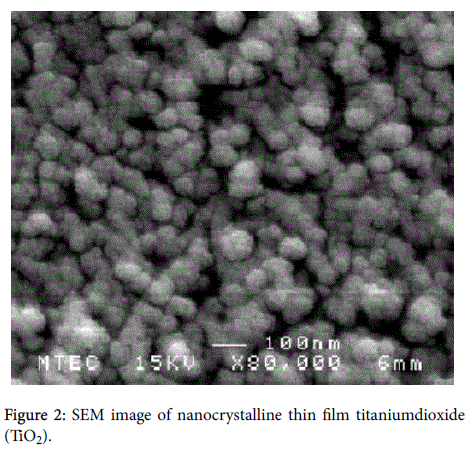
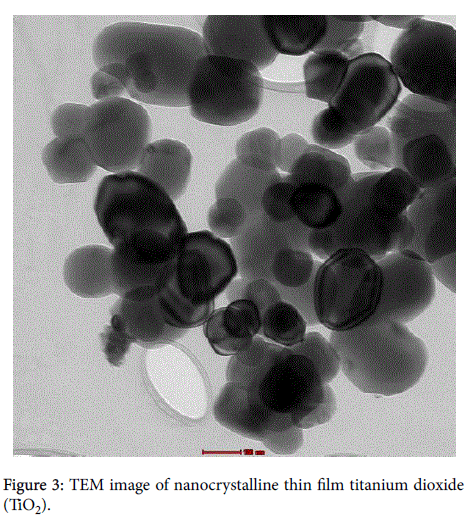
X-ray diffraction (XRD) was used to identify the phase formation. It was found that the phase of titanium dioxide (TiO2) was anatese structure and diffraction peaks were observed at 2θ=19°, 28.6°, 36.3°, and 48.5° show in Figure 1. Scanning electron microscopy (SEM) with magnification of X80,000 and transmission electron microscopy (TEM) images of the thin film Nano crystalline and solid state polymer electrolytes structure with the size of 100 nm to 200 nm are shown in Figures 2 and 3, respectively, which indicated a very small titanium dioxide (TiO2) nanoparticles.
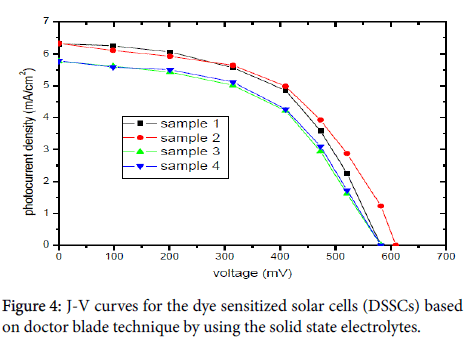
Figure 4 and Table 1 show the photocurrent-voltage characteristics curves (J-V) of dye sensitized solar cells (DSSCs) based on doctor blade technique by using the solid state electrolytes. An overall light to electrical energy conversion efficiency (?) of 2.49%, 2.56%, 2.16% and 2.18% for sample 1, 2, 3 and 4 respectively.
| Solid state electrolytes | Jsc (mA/cm2) | Voc(V) | FF | ? (%) |
|---|---|---|---|---|
| Sample 1 | 6.31 | 0.582 | 0.542 | 2.49 |
| Sample 2 | 6.32 | 0.609 | 0.531 | 2.56 |
| Sample 3 | 5.76 | 0.581 | 0.516 | 2.16 |
| Sample 4 | 5.76 | 0.582 | 0.521 | 2.18 |
Table 1: Photoelectrochemical data of the dye sensitized solar cells (DSSCs).
Conclusion
In this work, The dye sensitized solar cells (DSSCs) based on doctor blade technique by using the solid state electrolytes yielded of sample 1, 2, 3 and 4 an open-circuit voltage (Voc), of 0.582 V, 0.609 V, 0.581 V, 0.582 V, the short-circuit current density (Jsc), of 6.31 mA/cm2, 6.32 mA/cm2, 5.76 mA/cm2, 5.76 mA/cm2, fill factor (FF), of 0.542, 0.531, 0.516, 0.521, and overall light to electrical energy conversion efficiency (%) of 2.49%, 2.56%, 2.16% and 2.18% respectively, under irradiation of 80 mW/cm2.
Acknowledgement
Authors wish to thank Prof. Dr. Udom Tipparach, department of physics, faculty of science, Ubon Rajathanee University and appropriate technology center sakon nakhon Rajabhat University.
References
- Kim JH, Kang MS, Kim YJ, Won J, Kang YS (2005) Poly (butyl acrylate)/NaI/I 2 electrolytes for dye-sensitized nanocrystalline TiO2 solar cells. Solid State Ionics 176: 579-584.
- O’regan B, Grfitzeli M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353: 737-740.
- Nazeeruddin MK, Kay A, Rodicio I, Humphry-Baker R, Muller E, et al. (1993) Conversion of light to electricity by cisX2bis (2,2’-bipyridyl-4,4’-dicarboxylate) ruthenium(II) charge-transfer sensitizers (X-Cl-,Br-,I-,CN-,and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115: 6382- 6390.
- Wu J, Lan Z, Wang D, Hao S, Lin J, et al. (2006) Quasi-solide state dye sensitized solar cells based gel polymer electrolytes with poly(acrylamide)-poly-(ethylene glycol) composite. J Photochem Photobiol 181: 333-337.
- Stergiopoulos T, Bernard MC, Hugot–Le Goff A, Fararas P (2004) Resonance micro- Raman spectrophoto electrochemistry on nanocrystalline TiO2 thin film electrodes sensitized by Ru (II) complexes. Coord Chem Rev 248: 1407-1420.
Citation: Santhaveesuk S, Santhaveesuk T, Kahattha C (2016) A Novel Development for Dye Sensitized Solar Cells Solid-state Electrolytes without the Use of a Clean Room. Innov Ener Res 5: 143.
Copyright: ©2016 Santhaveesuk S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 9799
- [From(publication date): 12-2016 - Dec 18, 2024]
- Breakdown by view type
- HTML page views: 9102
- PDF downloads: 697