Research Article Open Access
A Novel Approach for Treatment Patients with Multiple Sclerosis by Using DNA Polymerase
Sherif Salah*
Consultant of Clinical Pathology/Faculty of Vet Medicine, University of Cairo, Egypt
- *Corresponding Author:
- Sherif Salah Salah
Department of Clinical Pathology
Cairo University, Cairo, Egypt
Tel: 01005218981
E-mail: sherif64@mail.com
Received date: April 27, 2016; Accepted date: May 03, 2016; Published date: May 10, 2016
Citation: Salah S (2016) A Novel Approach for Treatment Patients with Multiple Sclerosis by Using DNA Polymerase. J Alzheimers Dis Parkinsonism 6:235. doi:10.4172/2161-0460.1000235
Copyright: © 2016 Salah S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Introduction: Multiple sclerosis (MS) is a complex inflammatory demyelinating disease of the central nervous system (CNS) with both genetic and environmental contributing factors. In recent years, increasing evidence has pointed to the potential role of fibrinolysis in the pathogenesis of MS. Based on hypotheses describing the aggressive autoimmune responses observed in MS patients, a result of impaired which results in impairment between t-PA and PA1-1 which are a key molecules in both fibrinolysis and extracellular proteolysis.
Aim of the study: The aim of the present study is to investigate the therapeutic potential of polymerase enzyme in modulating the changes occurring between levels of Tissue- type plasminogen activator (t-PA) and its inhibitor (PAI-1) in patients with multiple sclerosis.
Patients and methods: A pilot study was carried out on a total of twenty-one patients (17 females, 4 males; aged 22-46 years) with demyelination suggestive of MS and clinically silent T2 brain lesions on magnetic resonance imaging (MRI). All of the examined patients showed the same clinical symptoms of MS and consented to take the novel therapy in the form of subcutaneous injection of 0.1 cc of DNA polymerase enzyme twice daily for 24 weeks. At the beginning of this study and at the end of therapy the plasmatic levels of PAI-1and t-PA were measured by ELISA and their values were expressed in ng/mg of protein.
Results: All patients showed a significant association between the decreased levels of PAI-1and the disappearance of annualized relapse rate (ARR), disability progression, and magnetic resonance imaging (MRI) activity.
Conclusion: From this study we conclude that DNA polymerase is viable therapeutic option in patients with MS.
Keywords
Multiple sclerosis; MS; MRI; T-PA; PA1-1
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the CNS. It results in neurological impairments that range from sensory defects to difficulties in movement and paralysis. The cause of MS remains unknown, although viral, environmental, and genetic factors have been proposed to contribute to its development [1]. In recent years, increasing evidence has pointed to the potential role of fibrinolysis in the pathogenesis of MS [2]. Specially the characteristic inflammation, focal demyelination, and axonal degeneration in MS occurring after disruption of the blood-brain barrier (BBB) and entry of serum proteins, including fibrinogen, into the CNS [3]. The key molecules in the PA system are tissue-type plasminogen activator (t- PA) and its inhibitor (PAI-1). Due to the formation of t-PA and inhibitor (e.g. PAI) complexes, the fibrinolytic potential in demyelinating MS lesions is greatly diminished. The limited availability of t-PA resulting from the formation of the t-PA/PAI- 1 complex is assumed to reduce the ability of t-PA receptors to produce plasmin, which further diminishes the fibrinolytic capacity in MS lesions, possibly avoiding increased axonal fibrin deposition and neurodegeneration [4]. This could also help to remove fibrin deposits, which are then cleared through internalization by macrophages [5]. In this study we investigate the effect of DNA polymerase in regulating fluctuations in the Levels of PAI-1and t-PA in blood samples, and its effect in bringing improvements of MS activity.
Patients and Methods
Twenty-one patients (17 women, 4 men; aged 20-46 years) with demyelinating suggestive of MS and clinically silent T2 weighed. MRI revealed two or more lesions in the central nervous system of all patients in this study. All of these patients have not received previously immunosuppressive therapy and none of them showed any laboratory evidence of diabetes mellitus, liver, renal or metabolic disorders. All patients fulfilled the criteria for clinically definite MS [inclusion criteria: patient suffering from either primary or secondary progressive multiple sclerosis with relapse, Patient with EDSS score of [1.0 to 8.0 and has at least two clinically silent lesions on the T2-weighted MRI scan, with a size of at least 3 millimeter (mm)]. We excluded patients who had a history of taking any immune modulatory or immunosuppressive therapy, oral or systemic corticosteroids within 10 days prior to study Day and patients with liver diseases, kidney and cardiac disease, such as angina, congestive heart failure or arrhythmia.
The patients were classified into three groups, according the numbers of lesions in white matter and the disease severity using the Multiple Sclerosis Severity Score (MSSS), which corrects the Expanded Disability Status Scale (EDSS). Group A 10 patients (9 female and 1 male) included patients with relapsing-remitting (RR) or primary progressive (PP) score ranging from 1.5 to 3 with one to two lesions, Group B 6 patients (4 female and 2 male), with secondary progressive (SP) course score ranging from 3 to 5 with two to three lesions and Group C 5 patients (4 female and one male) with score ranging from 5 to 7.5 with more than three lesions by using MRI scans. PAI-1plasma level.
Injection Material
DNA polymerase enzyme (available in experimental laboratories as DNA polymerase) in solution vial form which contains 6 ml/ each. Each patient was advised to take 6 vials during the duration’s therapy. This was given as subcutaneous injection, 0.1 cc twice daily for 24 weeks. All of the patients consented to this therapy, and two consecutive serum samples were obtained from all of them. The serum samples were analyzed before the beginning of the study and two Weeks after the end of the therapy. ELISA kit for PAI-I (American Diagnostic, Greenwich, CN, USA (and t-PA (Techno clone) were used, plasma samples were cold-centrifuged, immediately separated and stored in -70°C, and the quantities were measured using t-PA and PAI- 1 previously diagnostic kits, (normal values PAI-1 n: 2.2-11.2 ng/ml and t-PA (n: 0.5-4.2 ng/ml).
Quantitative Assay for PAI-1 and t-PA Level
First samples which collected before starting the therapy were assayed to measure the concentrations level of PAI-1and t-PA by comparing the absorbance of each well with a series of absorbance values obtained from known plasma concentrations of PAI-1, t-PA kits.
Statistical analysis was carried out using SPSS 21.0. Results are presented as percentage, mean ± SD. Continuous variables were compared using t test, and categorical variables were analyzed with χ2 test. Correlation analyses were performed using Pearson. In all statistics, two-sided tests were used and the results were considered statistically significant at P ≤ 0.05 and highly significant P ≤ 0.005. This is not a randomized controlled study.
Results
Study was carried on 21 patients 4 male (19.04%) and 17 female (80.96%) with clinical diagnosis of MS, their age ranged between 22 and 46 years with a mean of 33.57 years old (SD ± 7.65) (Table 1).
| Range | Mean ± SD | ||
| Age ( Years) | n=21 | 22-46 | 33.57 ± 7.65 |
| Number % | % | ||
| Sex | Male | 4 | 19.04 |
| Female | 17 | 80.96 |
Table 1: Descriptive data of the studied patients.
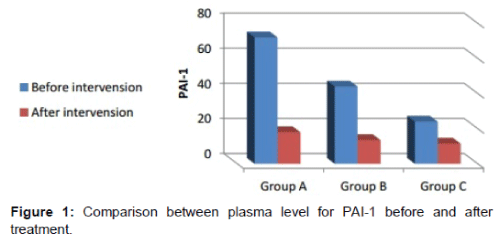
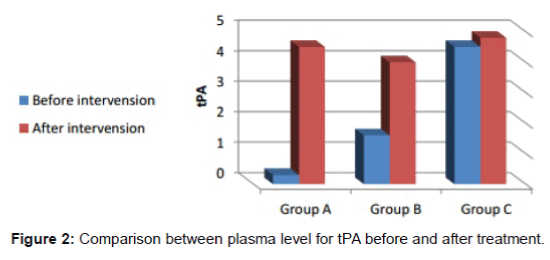
The first plasma sample prior starting in (group A) their PAI-1 levels showed 6 higher folds 72 ± 66 ng/ml, ;(group B) gave 4 higher folds 44 ± 39 ng/ml and (group C) demonstrated 2 higher fold 24 ± 21. While the t-PA levels is 0.3 ± 0.2 ng/ml in group A, 1.6 ± 0.4 ng/ml in group B; and 4.5 ± 1.2 ng/ml in group C (Table 2).
| Group A | Group B | Group C | ANOVA | ||
| (10 Patients) | (6 Patients) | (5 Patients) | (Sig) | ||
| (9F and 1M) | (4F and 2M) | (4F and 1 M) | |||
| PAI-1 Level | Before Therapy | 72+66ng/ml | 44+39ng/ml | 24+21ng/ml | 0.242(NS) |
| After Therapy | 18 ± 1.2 ng/ml | 13.5 ± 1.5 ng/ml | 11.5 ± 2.3 mg/ml | 0.000(HS) | |
| p (sig.) | 0.018 (s) | 0.08 (ns) | 0.222 (ns) | ||
| tPA level | Before Therapy | 0.3 ± 0.2 ng/ml | 1.6 ± 0.4 ng/ml | 4.5 ± 1.2 ng/ml | 0.000(HS) |
| After Therapy | 4.5 + 0.5 ng/ml | 4.0 + 0.6 ng/ml | 4.8 + 0.7 ng/ml | 0.09 (NS) | |
| p (sig.) | 0.0001 (HS) | 0.0001 ( HS) | 0.642 (NS) |
Table 2: Comparison between plasma level for t – PA and PAI – I before and after treatment.
The second samples were collected two weeks after last injection, and the collected data revealed that PAI-1level in group A decreased significantly after intervention to become 18 ± 12 ng/ml, group B 13.5 ± 1.5 ng/ml and 11.5 ± 2.3 ng/ml in group C, The t- PA levels increased to 4.5 ± 0.5 ng/ml in group A, 4.0 ± 0.6 ng/ml in group B and 4.8 ± 0.7 ng/ml in group C, this increase was statistically significant in groups A & B while it was non-significant in group C.
A comparison between plasma level for PAI-1 before and after treatment is shown in Figure 1, and a comparison between plasma level for tPA before and after treatment is shown in Figure 2.
The outcome results measured in group A showed a change from 3 to 0 using EDSS scale, with reduction in number and volume of lesions and no new or enlarging lesions on T2 weighted MRI image.
In Group B change from 5 to 1 with reduction in number and volume of lesion and no new or enlarging lesions on T2-weighted MRI image. In Group C change from 8 to 3 with reduction in number and volume of lesion and no new or enlarging lesions on T2-weighted MRI image, no any side effect or adverse reactions recorded during duration therapy in all patients.
Discussion
Multiple sclerosis (MS) is a complex inflammatory demyelinating disease of the central nervous system (CNS). Emerging evidence from the fields of neuroscience, immunology, and vascular biology have aimed the spotlight on fibrin and the fibrinolytic system for their pleiotropic functions in neurological diseases in its pathogenesis. Studies have proved that DNA polymerase is a vital enzyme for the regulation of multiple physiological cellular functions such as DNA repair, gene transcriptions, and cell cycle progression, cell death, chromatin function, and genomic stability [6]. It is not surprising that cells in all organisms contain multiple highly specialized DNA polymerases; the majority of it has been recently discovered. One of the main tasks for polymerase is to repair the opposite template lesions by a process known as translation synthesis. Extracellular proteolysis represents a potent and irreversible mechanism modulating the extracellular matrix and tissue remodeling, which can affect the breakdown of the BBB. Extracellular proteolytic enzymes have been implicated as important factors in demyelinating neuro inflammatory disorders such as MS [7]. The enzymes of the plasminogen activators/plasmin (PA) system are involved in both fibrinolysis and extracellular proteolyses. Plasminogen activator inhibitor (PAI-1) is a potent inhibitor of fibrinolysis that functions in the regulation of the plasmin- based pericellular proteolytic cascade [8]. PAI-1 also serves to regulate cell migration through binding matrix protein, such as vitronectin and heparin. PAI-1 is synthesized in endothelial cells and its release may be stimulated by the onset of inflammation. More reports recorded elevation of the PAI- 1 concentrations in patients with multiple sclerosis, encephalitis, viral meningitis, and leukemia [9].
Tissue plasminogen activator (t-PA), a neuronal as well as a key fibrinolytic enzyme, is found in high concentration in demyelinated axons in multiple sclerosis lesions together with fibrinogen deposits .t-PA is also present in high concentration in neurons, where upon activation, it has been found to have a role in neuronal development and synaptic remodelling [10]. The roles of plasminogen activators (t- PA) and plasminogen activator inhibitors (PAI- 1) in the pathogenesis of neurological disease have been previously suggested [11]. We believe that the growing of human brain lesions in multiple sclerosis patients results from excessive fibrin deposition in nerve cells which is being reversibly cleared with action of t-PA. This phenomenon could be considered a defensive mechanism, but with time, this reversible continuous dynamic action triggers the formation of antibodies against t-PA that interferes with the action of t-PA, thus paving the way for extra expression for PAI-1system. It is well known that DNA is replicated and repaired by a family of enzymes called DNA polymerases The major function of DNA polymerases is to replicate the genome and thus to allow transmission of genetic information from one generation to the next. Added to replicating DNA, polymerases help to maintain the integrity of the genome by participating in various modes of DNA repair. Polymerases involved also in chromosome replication [12]. Carrying such important functions, it would be very interesting to establish a new role for DNA polymerases Outside of chromosome replication and repair, in processes such as enhancement of myelin regenerations in nerve cells In this study we introduce DNA polymerase as a novel therapeutic intervention in vial form to be injected subcutaneously to restore the levels of PAI-I to their normal values. PAI-1 may acts as a “molecular switch” within the extracellular matrix that regulates the progression of cellular migration [13]. The clinical benefits of DNA polymerase treatment in MS patients may, in part, be a result of DNA polymerase’s ability to control the cellmatrix interaction. This regulatory mechanism represents a protective mechanism to remove fibrin deposits, which exacerbate axonal injury and promote regeneration by activating the growth factors. Raised plasma concentrations of PAI-1 have been reported in infections and during pregnancy, whereas increased cerebrospinal fluid (CSF) concentrations have been reported in some neurological diseases [14]. The roles of plasminogen activators and plasminogen activator inhibitors PAI-1in the pathogenesis of neurological disease and tumor development have been suggested.
The results obtained by this study showed a positive relationship between active MS processes and elevated plasma PAI-1levels, as well as a direct relation between the injections of DNA polymerase, decreasing the levels of PAI-1 the regression in numbers of brain lesions and improving their ability levels, this will explain the role of this therapy for treating relapsing remitting MS which will ultimately lead to a new generation of drugs as a regenerative medicine The current therapies, such as interferon beta, aim to suppress the immune attack that demyelinates nerve fibers. However, they are only partially effective and have significant adverse side effects. This study and its promising results could be a step towards a more reliable solution for a global problem. On the other hand, we have to confess that there was a limitation concerning our study due to the small sample size which emerged from the limited cases of MS found in Cairo and also the restricted budget as well. We emphasize that a further extended and more elaborate studies are needed to evaluate the benefits and values of our novel therapy.
Acknowledgments
Special thank for centre of neurology in El rehab Hospital, Egypt for its skillful assistance. This study was completely funded by the chairmen of El rehab specialized Hospital and patent office of Egypt.
References
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2, Sawcer S, Hellenthal G, Pirinen M, et al. (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214-219.
- Zivkovia M, A Ceizmarevia N, Lovrea L, Klupka-Saria I, Stankovia A, et al. (2014) The role of TPA I/D and PAI-1 4G/5G polymorphisms in multiple sclerosis. Dis Markers 2014: 362708.
- Baranzini SE, Nickles D (2012) Genetics of multiple sclerosis: swimming in an ocean of data. CurrOpinNeurol 25: 239-245.
- Gveric D, Herrera B, Petzold A, Lawrence DA, Cuzner ML (2003) Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain 126: 1590-1598.
- Gveric D, Herrera BM, Cuzner ML (2005) tPA receptors and the fibrinolytic response in multiple sclerosis lesions. Am J Pathol 166: 1143-1151.
- Prakash S, Johnson RE, Prakash L (2005) Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem 74: 317-353.
- East E, Baker D, Pryce G, Lijnen HR, Cuzner ML, et al. (2005) A role for the plasminogen activator system inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis. Am J Pathol 167: 545-554.
- Stoop A, van Meijer M, Horrevoets AJ, Pannekoek H (1997) Molecular advances in plasminogen activator inhibitor 1 interaction with thrombin and tissue-type plasminogen activator. Trends Cardiovasc Med 7: 47-51.
- Sutton R, Keohane ME, VanderBerg SR, Gonias SL (1994) Plasminogen activator inhibitor-I in the cerebrospinal fluid as an index of neurological disease. Blood Coagul Fibrinolysis 5: 167-171.
- Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, et al. (2000) Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci 12: 1002-1012.
- Gveric D, Cuzner ML, Newcombe J (1999) Insulin-like growth factors and binding proteins in multiple sclerosis plaques. NeuropatholApplNeurobiol 25: 215-225.
- Franklin MC, Wang J, Steitz TA (2001) Structure of the replicating complex of a pol alpha family DNA polymerase. Cell 105: 657-667.
- Stefansson S, Lawrence DA (1996) Theserpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 383: 441-443.
- Akenami FO, Koskiniemi M, Färkkilä M, Vaheri A (1997) Cerebrospinal fluid plasminogen activator inhibitor-1 in patients with neurological disease. J ClinPathol 50: 157-160.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11382
- [From(publication date):
June-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10482
- PDF downloads : 900