A Non-Surgical Foot-Worn Device for Patients with Hip Osteoarthritiswho Meet Surgical Criteria for Joint Replacement: A Retrospective Auditon NHS Patients
Received: 31-Jan-2023 / Manuscript No. jnp-24-126399 / Editor assigned: 02-Feb-2023 / PreQC No. jnp-24-126399(PQ) / Reviewed: 16-Feb-2023 / QC No. jnp-24-126399 / Revised: 21-Feb-2023 / Manuscript No. jnp-24-126399(R) / Published Date: 28-Jan-2023 DOI: 10.4172/2165-7025.1000673
Abstract
Purpose: To investigate the effect of a personalised, home-based, biomechanical foot-worn device on the referral rates for secondary care amongst patients with Hip Osteoarthritis (OA) who meet the clinical criteria for joint replacement.
Methods: One hundred and fifty-seven patients with hip OA participated in a commercial programme by Circle Integrated Care (CIC) offering innovative musculoskeletal rehabilitation services and pathway management. Patients were calibrated with a foot-worn biomechanical device that re-distributes loads via centre of pressure manipulation and generates perturbation to train proprioception. Patients are personally calibrated based on their gait profile and symptoms, then receive a home-based treatment plan that typically includes daily wear for 30-60 minutes while going about daily activities. Patients were reassessed clinically after six months, 1, 2 and 3 years. A mixed linear model was used to examine clinical changes over time.
Results: There were thirty-one (19.7%) referrals for secondary consultation. The mean days to referral was 310.5 (SD=273.8) days. 67% of all referrals occurred during the first year of treatment. Significant improvements were calculated for all clinical outcomes.
Conclusion: The results of the study suggest a significant clinical improvement among patients who failed core therapies and meet the criteria for joint replacement, with over 80% of the patients avoiding it for at least three years. This intervention should be considered as an additional non-surgical option for patients with hip OA who meet surgical criteria for joint replacement.
Keywords
Hip osteoarthritis; Total hip replacement; Non-surgical interventions; Biomechanics; Pain; Function
Introduction
Hip Osteoarthritis (OA) is one of the leading causes of disability and chronic pain among adults, affecting millions worldwide. Around 10% of adults above 45 years old have radiographic and symptomatic hip OA [1] and according to recent statistics, the prevalence of hip OA is on the rise [2]. Some estimates conclude that one in four people will develop symptomatic hip OA by the age of 85, with older age and female sex being dominant risk factors [3]. While it is less prevalent than knee OA, evidence on the long-term prognosis of hip OA suggests that patients are at a significantly higher risk of progressing to total joint replacement and have a shorter window of opportunity for conservative treatment between diagnosis and surgery [4]. Current data from the National Joint Registry suggest that there are 85K primary total hip replacements (THRs) in the UK annually. With the aging population and the increase in hip OA prevalence, it is projected that by 2060 the number of THR surgeries in the UK will surge by almost 40% compared to 2018 [5]. Taken together, these trends highlight hip OA as a major healthcare concern and call for an effective management strategy to improve patients' quality of life and reduce the burden on healthcare systems.
Non-surgical treatments of hip OA focus on alleviating symptoms of pain and stiffness and maintaining function and generally include patient education, exercise, and use of pain medication such as nonsteroidal anti-inflammatory drugs. Long-term prospective studies show that patients with symptomatic hip OA are likely to experience a rapid deterioration in pain and function, and a high proportion of them progress to surgery within three years since their initial consultation in the clinic [4,6-8]. This trend suggests that current conservative management of mild to moderate hip OA is not optimal and highlights the need for treatment that provides pain relief and targeted mobility and functional rehabilitation whilst simultaneously addressing the underlying mechanisms of the disease. In the past Febade accumulating evidence has demonstrated the beneficial effect of a noninvasive biomechanical intervention for knee and hip osteoarthritis [9- 14]. This intervention uses a customised, shoe-like device that shifts forces around the lower limb joints to alleviate pain while introducing controlled perturbation to train proprioception. The National Institute of Clinical Excellence (NICE) recently recommended the intervention as a safe, clinically effective and cost-saving non-invasive treatment for patients with severe knee OA who do not want surgery [15].
Circle Health Group is the UK's leading independent provider of hospital services. Part of this group, Circle Integrated Care (CIC), offers innovative musculoskeletal rehabilitation services and pathway management. Circle has been implementing this biomechanical intervention since 2015. Currently, the service is provided in clinical practices in Bedfordshire and Greenwich, covering a population of over 780,000. The current study aims to report our six-year experience utilising this intervention and monitor the rates of receiving referrals to secondary care consultation amongst NHS patients with hip OA.
Methods
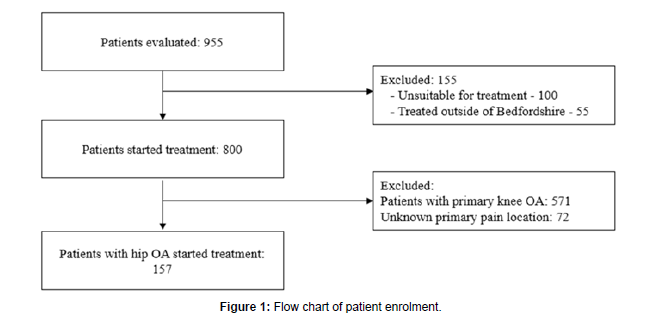
This was a retrospective analysis of 955 patients evaluated between October 2015 and March 2020. Of them, 800 patients began treatment, 100 were found unsuitable (for safety reasons such as poor balance) during the initial consultation, and 55 were treated outside of Bedfordshire and were not included in this analysis because they started treatment years later than the rest of the group. Of the 800 patients, 571 had primary knee OA and were not included in the analysis. Seventytwo patients were missing information about their primary pain area and were also not included in the analysis. One hundred and fifty-seven (N=157) patients met the criteria for orthopaedic referral as set out by NHS commissioners and managed by CIC as an integrated NHS provider (Figure 1).
Inclusion criteria were patients who met the surgical threshold for orthopaedic referral: radiological evidence of moderate to severe hip OA, and whose symptoms have failed to improve from conservative management (physiotherapy, activity modification, weight management, and non-steroid anti-inflammatory medication). Exclusion criteria were patients suffering from uncontrolled inflammatory conditions (e.g., Rheumatoid Arthritis); Patients who received a visco-supplementation/corticosteroid injection three months before treatment initiation (although exceptions could be considered, e.g., if the patient would benefit from both interventions); Patients suffering from neuropathic arthropathy (Charcot's joint); Patients exhibiting a lack of physical or mental ability to perform or comply with the treatment; Patients with a history of pathological osteoporotic fracture; Patients whose main complaint is another lower limb joint other than the hip and; Patients who are unsafe to participate in the treatment and fail to pass the balance protocol at the initial consultation (balance screening tool).
Once referred into the service, specially trained physiotherapists assessed patients and the suitable ones were enrolled in the programme. All patients who started treatment signed a consent acknowledging that their data might be used for research purposes while maintaining their privacy. Data was stored on a dedicated clinical system that meets privacy regulations. Patient is given a unique identifier and no additional identifying information (i.e. name, date of birth, contact details etc.) is provided. NHS Research Ethics Committee (REC) approval was not required under the UK Policy Framework for Health and Social Care Research as this data is unidentifiable. Furthermore, Health Research Authority approval is also not required for this research database.
Intervention
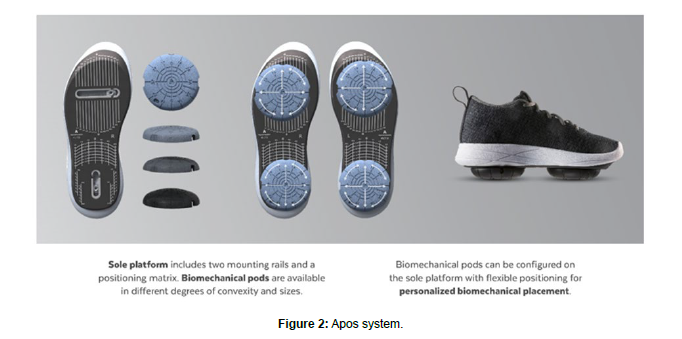
All patients received a personalised, non-invasive, home-based, biomechanical treatment to alleviate hip pain and improve function (AposHealth®). The device uses a shoe as an interface to attach two convex pods to the plantar surface of the sole (Figure 2). A speciallytrained physiotherapist calibrates the device based on a treatment methodology that includes an assessment of gait patterns, symptoms, and physical examination. The clinician calibrates the devices individually to the patient and positions the convex pods to reduce pain in the hip whilst walking. Adjusting the location of the pods changes the centre of pressure (COP) and the ground reaction force (GRF) vector with the aim of modifying the symptoms experienced on the area immediately [16-18]. The convex nature of the elements induces a level of controlled disturbance to gait and posture (i.e., perturbation) and generates neuromuscular training [19]. For example, in a typical patient with hip OA, following preliminary intake, a clinical and functional test is done to determine gait abnormalities and compensations. The physiotherapist observes the coronal/frontal plane as the patient walks to and from the clinician and calibrates the device to a neutral position for the specific patient. The neutral position is defined by minimal inversion/eversion of the foot during the stance phase. Once a neutral position is determined, the clinician will shift the posterior pod medially and posteriorly and the anterior pod will maintain in a neutral position or a slight lateral shift. The final adjustment of the device is determined when the patient reports a reduction in pain. Once the initial consultation and device calibration is completed, patients receive a home-based treatment plan. This includes wearing the customised device for approximately 20 minutes daily while doing regular tasks at home or work. Typically, patients gradually increase the device wear time for up to three hours per day indoors. Some patients will be encouraged to add outdoor walking depending on their progress and goals. In addition, patients are advised to return to follow-up appointments to re-calibrate the device and adjust the treatment plan as needed. During each visit, patients were asked to complete Patient Reported Outcomes Measure (PROM) and underwent a computerized gait test to monitor changes over time.
Outcomes
The primary outcome measure was the referral rates to secondary care consultation, including the service type and sub-service classification, between the commencement of treatment and the audit date (February 2022). Secondary outcome measures included two patient-reported outcome measures (PROMS) and a computerised gait test. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [20] questionnaire and the OHS [21] were used to assess changes in pain and function. The WOMAC questionnaire contains 24 Visual Analogue Scale (VAS) questions that can be divided into three sub-categories (Pain, Functional Limitation, and Stiffness). Results range between 0-100mm, in which 0mm indicates no pain or limitation in function and 100mm indicates the most severe pain or limitation in function. The OHS was developed and validated for use with individuals undergoing hip arthroplasty and measured outcomes following rehabilitation of patients with hip OA. It contains 12 Likert Scale questions. Results range from 0-48, where 0 reflects the worst condition and 48 is the best [20,21].
A computerised spatiotemporal gait assessment was used to assess gait velocity (cm/s) using the OptoGait system (Version 1.11) [22]. Patients walked barefoot at a self-selected speed over a four-meter measurement area, with two meters before and after to allow for sufficient acceleration and Deceleration time outside the measurement area.
All patients were asked to complete PROMs and conduct a computerised gait assessment during their first visit and at each followup appointment. The rates of referral to secondary care consultations were verified by querying the electronic system.
Statistical analysis
Data were analysed with IBM SPSS statistics software version 28.0. (SPSS Inc. Headquarters, 233 S. Wacker Drive, 11th floor Chicago, Illinois 60606, USA). The significance levels were set at P<0.05. Data were presented as mean and standard deviation for continuous variables and as frequency and percentage for categorical variables. We used a linear mixed model for three continuous outcomes (WOMAC-Pain, WOMAC-Function and velocity) with up to five time periods: baseline, six months, years one, two, and three years. The general linear mixed model is an acceptable approach in cases with missing information as it assesses the changes over time while accounting for missing data. In addition, Paired T-Test was used to examine the differences in OHS at baseline and 12 months, after confirming a normal distribution.
Results
One hundred and fifty-seven (157) patients with hip OA (103, 66% females) with a mean age of 63.3 (SD=9.7) years were included in this analysis. All patients completed at least one year of follow-up. The average days from treatment initiation to audit date was 1302.7 (SD=406.9) days per patient.
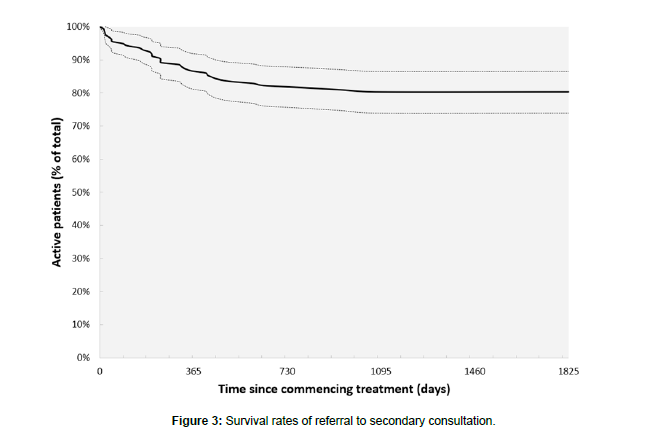
Overall, there were 31 (19.7%) referrals for secondary consultation. The mean days to referral was 310.5 (SD=273.8) days. 67% of all referrals occurred during the first year of treatment, 23% occurred during the second year, and 10% occurred during the third year. Figure 3 presents a survival graph of referral to secondary care.
The patients' compliance with follow-up appointments was high. The in-clinic attendance follow-up rates at six months, one year, two years, and three years follow-up appointments were 89%, 48%, 25%, and 11%, respectively. Significant improvement was seen in all clinical outcomes after six months and maintained for up to three years. We used a Linear Mixed Model to accommodate for missing values at different time points. The model imputed estimated means based on available data and prediction models. Results are summarised in Table 1. Overall, pain Decreased by 47.7% from an average (SE) of 53.9 (1.9) to 28.2 (5.8) at three years, P<0.001, F=8.887. Functional disability improved by 42.4% from an average (SE) of 54.8 (2.0) to 31.9 (6.1) at three years, p<0.001, F=6.445. Gait velocity increased by 26.7% from an average (SE) of 90.9 (1.5) to 115.2 (4.8) at three years, p<0.001, F=12.965. OHS increased by 19.0% from an average (SE) of 23.1 (0.9) to 27.5 (1.1) at 12 months, P<0.001, F=15.477. Gait velocity has increased by 24.8% from an average (SD) of 86.0 (17.0) to 107.3 (20.6) at three years, P=0.013, F=3.267.
| Baseline | 6 months | 1 Year | 2 Years | 3 Years | F | P | |
|---|---|---|---|---|---|---|---|
| WOMAC-Pain | 53.9 (1.9) [50.1-57.7] |
47.5 (2.0) [43.5-51.5] |
46.5 (2.7) [41.1-51.9] |
34.3 (3.7) [27.0-41.6] |
28.2 (5.8) [16.9-39.5] |
8.877 | <0.001 |
| WOMAC-Function | 54.8 (2.0) [50.8-58.8] |
48.4 (2.1) [44.1-52.6] |
48.8 (2.9) [43.2-54.5] |
37.1 (3.9) [29.5-44.8] |
31.9 (6.1) [20.0-43.8] |
6.445 | <0.001 |
| Velocity | 90.9 (1.5) [88.0-93.9] |
100.8 (1.5) [97.8-103.7] |
103.1 (2.1) [98.9-107.3] |
105.5 (2.9) [99.9-111.1] |
115.2 (4.8) [105.7-124.6] |
12.965 | <0.001 |
Table 1: Changes in WOMAC-pain, WOMAC-function and gait velocity. Results are presented as mean (SE) [95% CI].
There were no significant differences between those who progressed to secondary consultation and those who remained active in baseline characteristics, including age (p=0.197), gender (p=0.780), pain (0.643) and OHS (0.681).
Discussion
Hip OA is a complex and multifactorial disease with various factors contributing to its development and progression. Amongst them are age, genetics, previous injuries, obesity and biomechanics [23]. The biomechanical properties of the hip joint, including structural and soft tissue changes are altered, leading to abnormal loading patterns and mechanical stress that contribute to the onset and progression of hip OA [24,25]. Functional alterations may include changes in muscle strength and activation patterns, which can affect joint stability and loading patterns [26]. The biomechanical intervention used in this study aims to re-distribute loads on the lower extremity during locomotion via external manipulation of the centre of pressure. In addition, the biomechanical elements at the bottom of the shoe create perturbation which stimulates neuromuscular control. The treatment plan includes daily wear while going about daily activities, complying with functional rehabilitation recommendations, and ultimately helping the patient to acquire new motor patterns with reduced pain and improved function and quality of life. We speculate that this is the main reason for lower rates of referrals to secondary care consultation amongst patients with hip OA. The results of the current study support other studies that looked at the effect of gait retraining as a modality to treat patients with knee and hip OA [27]. However, the evidence for hip OA is scarce and further research is required [28].
The results of this study suggest that only 20% of the patients received a referral for secondary consultation. Paans reported a referral rate of 31% for secondary care amongst newly diagnosed patients with hip OA [29]. It is important to stress that patients in the current study were enrolled after exhausting core management of hip OA (i.e., exercise, education, physical therapy and pain relief medications) and met the clinical criteria for a THR and were therefore most severe in their pathology. McHugh et al., looked at the actual surgical rates of those who received a referral for secondary consultation and found that 50% of the patients that were referred for THR had it within 12 months [30]. That is in comparison to a 13% rate found in this study (first 12 months). It is reasonable to assume that the patient population in the study of McHuge resembles the population of the current study, suggesting >50% reduction in the rates of THR. The NHS national Tariff for THR ranges from £5,128 to £11,420. When applying a conversion to secondary care referral rate of 50% and 20% for those who are not treated with the device and those who are, respectively, for 100 patients referred to THR, the potential savings at 12 months will be between 189,736 to 422,540, not including the costs of the device. This has the potential to address the urgent need to reduce the long waiting list and, similar to NICE recommendations for patients with knee OA can provide effective interventions for patients who do not want to have surgery [15].
Some limitations should be acknowledged. First, this was a retrospective study with no control group, which limits the ability to control for potential confounding variables. That said, the results of the study reflect a real-world setting in which it was conducted, with patients receiving treatment in the clinical practices of Circle Integrated Care, demonstrating its feasibility in commercial settings. To the best of our knowledge, this is the first report of real-life experience with patients with hip OA and the effect on the rates of surgery/referral to secondary care. Further research is needed to support the finding of this study. Secondly, the examined intervention is primarily a home-based program. This study did not monitor and unified patient compliance, and there is a possibility that some patients did not comply with the program regularly. That said, this study looked at the rates of referral to secondary consultation, and reflect the “worst case” scenario (i.e. not all patients complied with the program regularly). We believe this is a true reflection of real-life that accounts for various scenarios that are not controlled and still demonstrate significant clinical improvement and low rates of referral to secondary care. Lastly, the study looked at patients with hip OA who meet surgical criteria and is limited in its generalisations to the entire hip OA population. Future studies should look at the effect of treatment in newly diagnosed patients with hip OA.
Conclusion
The examined intervention is a personalised, non-invasive, homebased, biomechanical device. The results suggest a significant clinical improvement among patients with hip OA who failed core therapies and met the criteria for joint replacement, with over 80% of the patients avoiding it for at least three years. These preliminary outcomes are promising and can help reduce the burden on healthcare systems and improve patients' quality of life. Therefore, we recommend that this intervention should be considered as an additional non-surgical option for patients with hip OA who meet surgical criteria for joint replacement.
Conflict of Interests
None to declare.
Human Ethics and Consent to Participate Declarations
Not applicable.
All patients who started treatment signed a consent acknowledging that their data might be used for research purposes while maintaining their privacy. NHS Research Ethics Committee (REC) approval was not required under the UK Policy Framework for Health and Social Care Research as this data is unidentifiable. Furthermore, Health Research Authority approval is also not required for this research database.
Author Contribution
All authors take full responsibility for the entire manuscript content, integrity of the data and the accuracy of the data analysis. Study Concept and Design: RB, LR, AP. Data collection and analysis: RB, LR. Analysis and Interpretation of Data: RB, LR, AP. Original Drafting of the Manuscript: RB. Critical Revision of the Manuscript: LR, AP. The author(s) read and approved the final manuscript.
References
- Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, et al. (2009) Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 36: 809-815.
- Swain S, Sarmanova A, Mallen C, Kuo CF, Coupland C, et al. (2020) Trends in incidence and prevalence of osteoarthritis in the United Kingdom: findings from the Clinical Practice Research Datalink (CPRD). Osteoarthritis Cartilage 28: 792-801.
- Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, et al. (2010) One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage 18:1372-1379.
- Dabare C, Le Marshall K, Leung A, Page CJ, Choong PF, et al. (2017) Differences in presentation, progression and rates of arthroplasty between hip and knee osteoarthritis: Observations from an osteoarthritis cohort study-a clear role for conservative management. Int J Rheum Dis 20: 1350-1360.
- Matharu GS, Culliford DJ, Blom AW, Judge A (2022) Projections for primary hip and knee replacement surgery up to the year 2060: an analysis based on data from The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Ann R Coll Surg Engl 104: 443-448.
- Gustafsson K, Kvist J, Zhou C, Eriksson M, Rolfson O (2022) Progression to arthroplasty surgery among patients with hip and knee osteoarthritis : a study from the Swedish BOA Register. Bone Joint J 104: 792-800.
- Svege I, Nordsletten L, Fernandes L, Risberg MA (2015) Exercise therapy may postpone total hip replacement surgery in patients with hip osteoarthritis: a long-term follow-up of a randomised trial. Ann Rheum Dis 74: 164-169.
- Gwynne-Jones JH, Wilson RA, Wong JMY, Abbott JH, Gwynne-Jones DP (2020) The Outcomes of Nonoperative Management of Patients With Hip and Knee Osteoarthritis Triaged to a Physiotherapy-Led Clinic at Minimum 5-Year Follow-Up and Factors Associated With Progression to Surgery. J Arthroplasty 35: 1497-1503.
- Drexler M, Segal G, Lahad A, Haim A, Rath U, et al. (2013) A non-invasive foot-worn biomechanical device for patients with hip osteoarthritis. Surg: Curr Res.
- Solomonow-Avnon D, Herman A, Levin D, Rozen N, Peled E, et al. (2017) Positive outcomes following gait therapy intervention for hip osteoarthritis: A longitudinal study. J Orthop Res 35: 2222-2232.
- Solomonow-Avnon D, Haim A, Levin D, Elboim-Gabyzon M, Rozen N, et al. (2016) Reduction of hip joint reaction force via medio-lateral foot center of pressure manipulation in bilateral hip osteoarthritis patients. J Orthop Res 34: 1762-1771.
- Miles C, Greene A (2020) The effect of treatment with a non-invasive foot worn biomechanical device on subjective and objective measures in patients with knee osteoarthritis- a retrospective analysis on a UK population. BMC Musculoskelet Disord 21: 386.
- Greene A, Miles C (2022) Surgery avoidance rates among total knee replacement candidates following a non-invasive biomechanical intervention: A retrospective cohort study. Journal of Orthopaedic Experience and Innovation.
- Reichenbach S, Felson DT, Hincapié CA, Heldner S, Bütikofer L, et al. (2020) Effect of Biomechanical Footwear on Knee Pain in People With Knee Osteoarthritis: The BIOTOK Randomized Clinical Trial. JAMA 323: 1802-1812.
- NICE MTG (2023) AposHealth for knee osteoarthritis.
- Haim A, Rozen N, Wolf A (2010) The influence of sagittal center of pressure offset on gait kinematics and kinetics. J Biomech 43: 969-977.
- Haim A, Wolf A, Rubin G, Genis Y, Khoury M, et al. (2011) Effect of center of pressure modulation on knee adduction moment in medial compartment knee osteoarthritis. J Orthop Res 29: 1668-1674.
- Solomonow-Avnon D, Herman A, Wolf A (2019) Mechanism of reducing knee adduction moment by shortening of the knee lever arm via medio-lateral manipulation of foot center of pressure: A pilot study. J Biomech 83: 143-149.
- Debbi EM, Wolf A, Haim A (2012) Detecting and quantifying global instability during a dynamic task using kinetic and kinematic gait parameters. J Biomech 45: 1366-1371.
- Hmamouchi I, Allali F, Tahiri L, Khazzani H, Mansouri LE, et al. (2012) Clinically important improvement in the WOMAC and predictor factors for response to non-specific non-steroidal anti-inflammatory drugs in osteoarthritic patients: a prospective study. BMC Res Notes 5: 58.
- Beard DJ, Harris K, Dawson J, Doll H, Murray DW, et al. (2015) Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 68: 73-79.
- Lee MM, Song CH, Lee KJ, Jung SW, Shin DC, et al. (2014) Concurrent Validity and Test-retest Reliability of the OPTOGait Photoelectric Cell System for the Assessment of Spatio-temporal Parameters of the Gait of Young Adults. J Phys Ther Sci 26: 81-85.
- Rees HW, Barba M (2020) AAOS Clinical Practice Guideline: Management of Osteoarthritis of the Hip. J Am Acad Orthop Surg 28: e292-e294.
- Astephen Wilson JL, Kobsar D (2021) Osteoarthritis year in review 2020: mechanics. Osteoarthritis Cartilage 29: 161-169.
- D'Souza N, Charlton J, Grayson J, Kobayashi S, Hutchison L, et al. (2022) Are biomechanics during gait associated with the structural disease onset and progression of lower limb osteoarthritis? A systematic review and meta-analysis. Osteoarthritis Cartilage 30: 381-394.
- Murphy NJ, Eyles JP, Hunter DJ (2016) Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv Ther 33: 1921-1946.
- Benn R, Rawson L, Phillips A (2013) Utilising a non-surgical intervention in the knee osteoarthritis care pathway: a 6-year retrospective audit on NHS patients. Ther Adv Musculoskelet Dis 15: 1759720X231187190.
- Rynne R, Le Tong G, Cheung RTH, Constantinou M (2022) Effectiveness of gait retraining interventions in individuals with hip or knee osteoarthritis: A systematic review and meta-analysis. Gait Posture 95: 164-175.
- Paans N, van der Veen WJ, van der Meer K, Bulstra SK, van den Akker-Scheek I, et al. (2011) Time spent in primary care for hip osteoarthritis patients once the diagnosis is set: a prospective observational study. BMC Fam Pract 12: 48.
- McHugh GA, Campbell M, Luker KA (2011) GP referral of patients with osteoarthritis for consideration of total joint replacement: a longitudinal study. Br J Gen Pract 61: e459-468.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Benn R (2024) A Non-Surgical Foot-Worn Device for Patients with Hip Osteoarthritis who Meet Surgical Criteria for Joint Replacement: A Retrospective Audit on NHS Patients. J Nov Physiother 14: 673. DOI: 10.4172/2165-7025.1000673
Copyright: © 2024 Benn R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2750
- [From(publication date): 0-2024 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2533
- PDF downloads: 217