Research Article Open Access
A New Chemiluminescent Enzyme Immunoassay for Plasma Tissue Factor Detection
Yuki Fujiwara1,2, Yoshikatsu Koga1, Kimiko Hasegawa3, Hiroya Fujii3, Tetsuji Yamaguchi3, Ryou Tsumura1,2, Masahiro Yasunaga1and Yasuhiro Matsumura1,2*
1Division of Developmental Therapeutics, Research Centre for Innovative Oncology, National Cancer Centre Hospital East, 6-5-1 Kashiwanoha, Kashiwa, Chiba, Japan
2Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba, Japan
3JSR Life Sciences, R&D Department Unit 1, 25 Miyukigaoka, Tsukuba, Ibaraki, Japan
- Corresponding Author:
- Yasuhiro Matsumura
Division of Developmental Therapeutics
Research Centre for Innovative Oncology
National Cancer Centre Hospital East
6-5-1 Kashiwanoha, Kashiwa, Chiba, Japan
Tel: 04- 7134-6857
E-mail: yhmatsum@east.ncc.go.jp
Received Date: October 23, 2014; Accepted Date: November 26, 2014; Published Date: December 03, 2014
Citation: Fujiwara Y, Koga Y, Hasegawa K, Fujii H, Yamaguchi T, et al. (2014) A New Chemiluminescent Enzyme Immunoassay for Plasma Tissue Factor Detection. Biosens J 3:111. doi: 10.4172/2090-4967.1000111
Copyright: © 2014 Fujiwara Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
High concentrations of tissue factor (TF) in the plasma may have a major role in the elevated risk of thrombosis in patients with malignant diseases. The quantification of plasma TF concentrations in cancer patients may enable accurate assessments of the risk of thrombosis during the process of cancer progression. Whether the plasma TF concentration is associated with the tumor volume in pancreatic cancer in the field of cancer biology remains uncertain. Rat anti-human TF monoclonal antibodies were prepared in our laboratory and were used in a chemiluminescent enzyme immunoassay (CLEIA) for TF detection. In an in vitro experiment, the antibodies for human TF did not crossreact with mouse TF. Therefore, tumor-derived TF was specifically detected using a novel sensitive human TF detection system developed for this study. The plasma concentration of tumor-derived TF was positively correlated with the tumor volume in mice bearing the human pancreatic cancer cell line BxPC-3. Thus, the plasma TF concentration may be a useful tumor marker. These data warrant further preclinical and clinical investigations of our CLEIA system.
Keywords
Pancreatic cancer; Tumor marker; Tissue Factor (TF); Chemiluminescent Enzyme Immunoassay (CLEIA)
Introduction
Ever since Armand Trousseau reported thrombophlebitis as a complication of cancer in the 19th century, an association between cancer and hyper coagulability has been generally accepted [1,2]. Tissue factor (TF), a 47-kDa trans membrane glycoprotein, is essential for blood coagulation.TF is known to be over expressed not only on tumor cells but also on tumor stromal cells including tumor vascular endothelial cells in various human tumor tissues [3,4]. Thus, the high plasma concentration of TF observed in patients with malignant diseases may have a major role in the elevated risk of thrombosis in these patients. The quantification of plasma TF-bearing micro particles in cancer patients may enable an accurate assessment of the risk of thrombosis during the process of cancer progression [5]. Pancreatic cancer patients have a strong likelihood of developing cancer-associated thrombosis, compared with healthy volunteers [6]. However, whether the plasma TF concentration is associated with the tumor volume in patients with pancreatic cancer remains uncertain in the field of cancer biology
In this context, we have constructed a new chemiluminescent enzyme immunoassay (CLEIA) using an anti-human TF monoclonal antibody that we developed [7]. The CLEIA automated system is known to be more effective than conventional systems, such as enzymelinked immune sorbent assay (ELISA) [8]. Therefore, the plasma TF concentrations before and after tumor resection were evaluated in mice bearing BxPC-3 subcutaneous tumors using our CLEIA system.
Materials and Methods
Cell lines
The human pancreatic cancer cell lines BxPC-3 and MIA PaCa-2 (TF-negative cells [7]) were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and were cultured in Roswell Park Memorial Institute (RPMI) 1640 Medium (Wako, Osaka, Japan) with incubation at 37°C in a humidified atmosphere containing 5% CO2.
Antibodies and proteins
Recombinant human and mouse TF proteins, which are the extracellular domains of human and mouse TF without the signal peptide, were obtained using conventional synthesizing methods and Escherichia coli. Recombinant human TF was used to make the calibration curve. The anti-human TF monoclonal antibody (mAb)- producing hybridoma clones 1849 and 1006 were established in our laboratory [7].
Western blotting analysis
Horseradish per oxidase (HRP)-labeled anti-human TF 1849 mAb was prepared using Per oxidase Labeling Kit-NH2 (DOJINDO Molecular Technologies, Kumamoto, Japan), according to the manufacturer’s instructions. The BxPC-3 cells and tumor tissues were lysed in whole extraction buffer (20 mM HEPES-NaOH, 0.5% NP-40, 15% glycerol) with a protease inhibitor cocktail (Roche Diagnostics). The proteins (tumor lysate and plasma) were immune precipitated using anti-human TF immune beads. The proteins were separated using sodium do decyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and heat-shocked conditions, and then electrophoretically transferred to polyvinylidenedifluoride membranes (Bio-Rad, Hercules, CA, USA) using the Trans-Blot Turbo transfer machine (Bio-Rad). The membranes were blocked overnight at 4°C with N102 blocking reagent (Nichiyu, Tokyo, Japan) and then incubated in the presence of 0.5 pg/mL HRP-labeled anti-human TF 1849 mAb in Can Get Signal solution 2 (TOYOBO) for 3 hours at 37°C. Subsequently, the membranes were washed with TBS-T. Finally, the proteins on the membrane were visualized using ECL prime (GE Healthcare, Piscataway, NJ) as the substrate. The membrane was analyzed using a Chemi Doc imager (Bio-Rad).
Commercially available ELISA kit for TF detection
The ELISA kit was purchased from Assay Pro (MO, USA) and was used according to the manufacturer’s instructions. The sensitivity of this kit was compared with our CLEIA method described below.
CLEIA for TF detection
A novel immune bead was used to establish our CLEIA system for TF detection. Magnosphere MS300/Carboxyl (JSR Life Sciences) was conjugated with anti-human TF 1849 mAb (anti-human TF immune beads). Horseradish per oxidase (HRP)-labeled anti-human TF 1006 mAb was then prepared using Per oxidase Labeling Kit-NH2 (DOJINDO Molecular Technologies), according to the manufacturer’s instructions. Anti-human TF immune beads as a capturing antibody (0.05%, 50 μL) and samples (100 μL, conditioned media and plasma) were added to a 96-well flat-bottom white polystyrene plate (Corning, NY, USA) and then the samples were incubated for 30 min at room temperature. Only the plasma samples were diluted 20 times with Can Get Signal solution 1 (TOYOBO, Osaka, Japan) to minimize nonspecific binding. The samples were then washed with Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBS-T) using Micro plate Washer Hydro Flex (Wako) and incubated with 100 μL of HRP-labeled anti-human TF 1006 mAb (1.0 mg/mL) as the detecting antibody for 30 min at room temperature. After washing, Super Signal ELISA Pico Chemiluminescent Substrate (Thermo, Waltham, MA) was applied to the samples, followed by incubation for 5 min at 37°C. Luminescent signals were detected using the Spectra Max paradigm (Molecular Devices, Tokyo, Japan). Noise intensity (N) represents the value of relative luminescent units (RLU) in the absence of TF protein. We defined the detection limit as the protein concentration at which the signal intensity (S) was twice the noise intensity (S/N=2).
Mouse xenograft model
Female BALB⁄ c nude mice (5 weeks old) were purchased from SLC Japan (Shizuoka, Japan). All the animal procedures were performed in compliance with the Guidelines for the Care and Use of Experimental Animals established by the Committee for Animal Experimental of the National Cancer Center. These guidelines meet the ethical standards required by law and also comply with the guidelines for the use of experimental animals in Japan. Mice were inoculated subcutaneously in the flank with 1×106 cells of BxPC-3. The length (L) and width (W) of the tumor masses were measured every 10 days, and the tumor volume was calculated as (L×W2)/2. At 30 days after inoculation with the BxPC- 3 cells, the tumor tissues were removed surgically. Blood was collected from the mouse tail vein into 500-μL tubes containing heparin sodium (Fuso Pharmaceutical Industries, Osaka, Japan). Plasma was separated from the blood by centrifugation at 2000xg for 5 min. The plasma samples were then stored at -80°C until use.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). All the results were statistically analyzed using Statcal QC software (The Publisher OMS, Saitama, Japan). A comparison of the TF values between the MIA PaCa-2- and BxPC-3-conditioned media was performed using a single-measure, simple-factorial ANOVA, followed by Student test. The chi-square test and the Spearman correlation coefficient were used to determine the significance of the associations between the plasma TF levels and the BxPC-3 tumor volume. A value of P < 0.05 was considered statistically significant.
Results
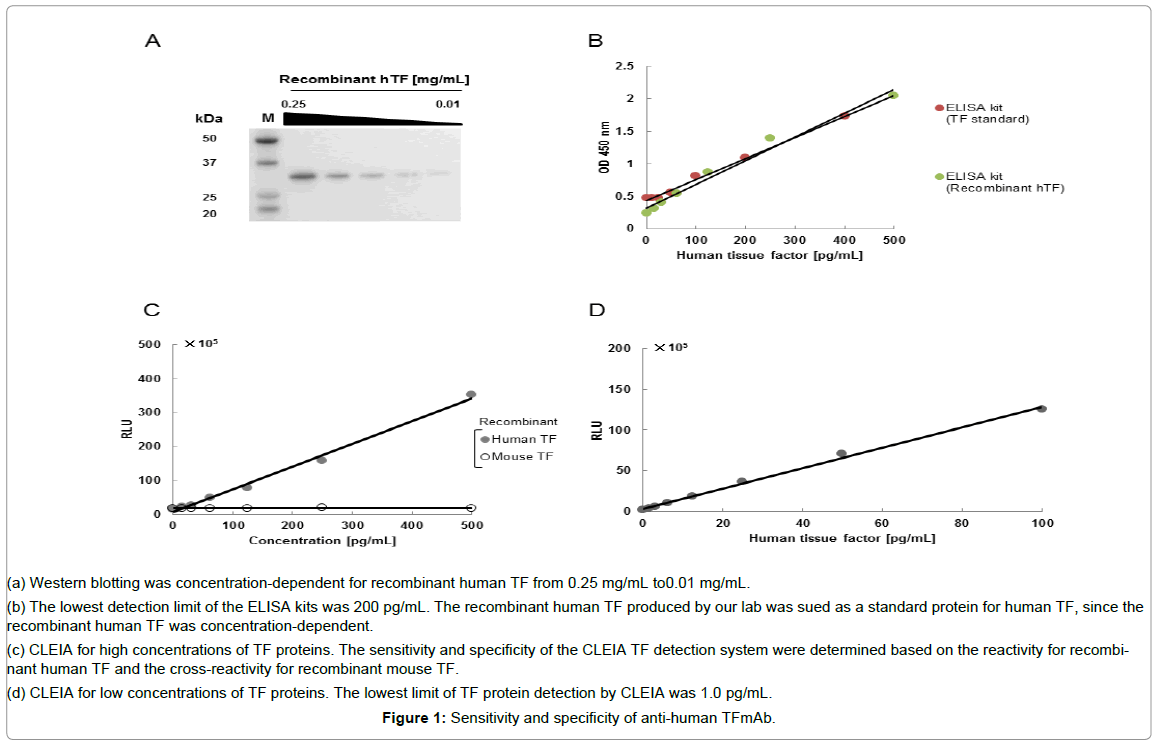
The specificity of anti-human TF 1849 mAb was clearly shown by the western blotting analysis (Figure 1A). In a commercially available ELISA kit, both the TF protein in the kit and our recombinant human TF protein produced a similar calibration curves (Figure 1B). The detection limit of the ELISA kit was 200 pg/mL (Figure 1B). Our CLEIA did not cross-react withmouse TF protein (Figure 1C).
Figure 1: Sensitivity and specificity of anti-human TFmAb.
(a) Western blotting was concentration-dependent for recombinant human TF from 0.25 mg/mL to0.01 mg/mL.
(b) The lowest detection limit of the ELISA kits was 200 pg/mL. The recombinant human TF produced by our lab was sued as a standard protein for human TF, since the recombinant human TF was concentration-dependent.
(c) CLEIA for high concentrations of TF proteins. The sensitivity and specificity of the CLEIA TF detection system were determined based on the reactivity for recombinant human TF and the cross-reactivity for recombinant mouse TF.
(d) CLEIA for low concentrations of TF proteins. The lowest limit of TF protein detection by CLEIA was 1.0 pg/mL.
To determine the sensitivity of CLEIA, a low concentration of recombinant human TF was used. The detection limit of the CLEIA method was determined to be 1.0 pg/mL (Figure 1D). The CLEIA system had a higher sensitivity than the ELISA kit. In addition, the ELISA system appeared to exhibit cross-reactivity against mouse TF (Table 1). Therefore, only the CLEIA system was used in subsequent analyses.
| system | detection | Primary antibody | Secondary body | Detection limit | Crossreactivity against mouse TF | |
|---|---|---|---|---|---|---|
| ELISA kit | Sandwich | Absorbance | Polyclonal | Stretavidin-biotinylated polyclonal | 200 pg/ml | + |
| CLEIA (our lab) | Sandwich | Chemi-luminescence | Monoclonal | monoclonal | 1.0 pg/ml | - |
Table 1: Enzyme immunoassay for TF
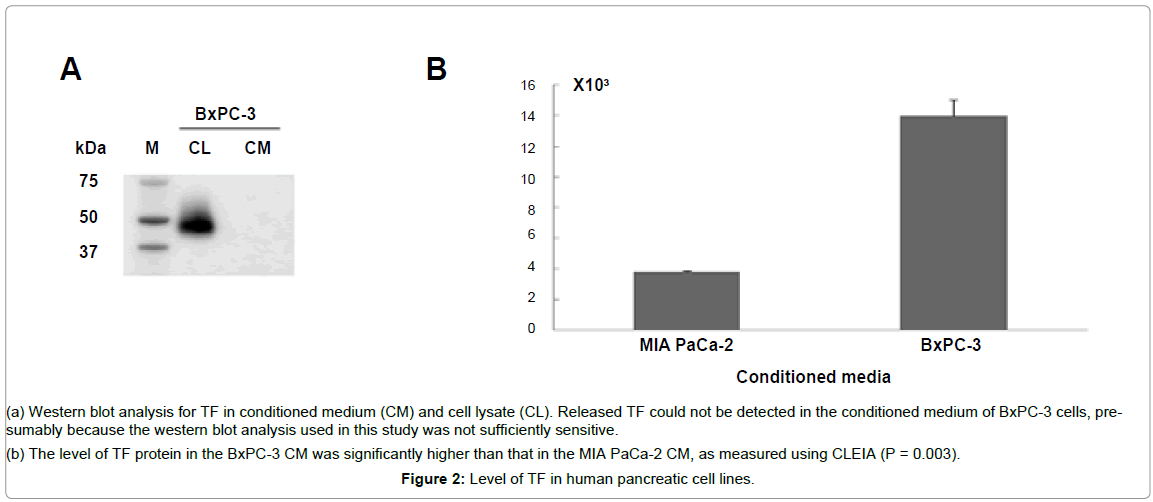
TF was detected in the BxPC-3 cell lysate using a western blotting analysis. However, the released TF could not be detected in the conditioned medium of the BxPC-3 cells, probably because the western blotting analysis used in this study was not sufficiently sensitive (Figure 2A). Therefore, CLEIA was used to detect the released TF from the tumor cells in the conditioned medium of BxPC-3. This assay revealed a significantly higher level of TF in the conditioned medium of the BxPC-3 cells than that in the conditioned medium of the TFnegativeMIA PaCa-2 cells (Figure 2B), (P = 0.003).
Figure 2: Level of TF in human pancreatic cell lines.
(a) Western blot analysis for TF in conditioned medium (CM) and cell lysate (CL). Released TF could not be detected in the conditioned medium of BxPC-3 cells, presumably because the western blot analysis used in this study was not sufficiently sensitive.
(b) The level of TF protein in the BxPC-3 CM was significantly higher than that in the MIA PaCa-2 CM, as measured using CLEIA (P = 0.003).
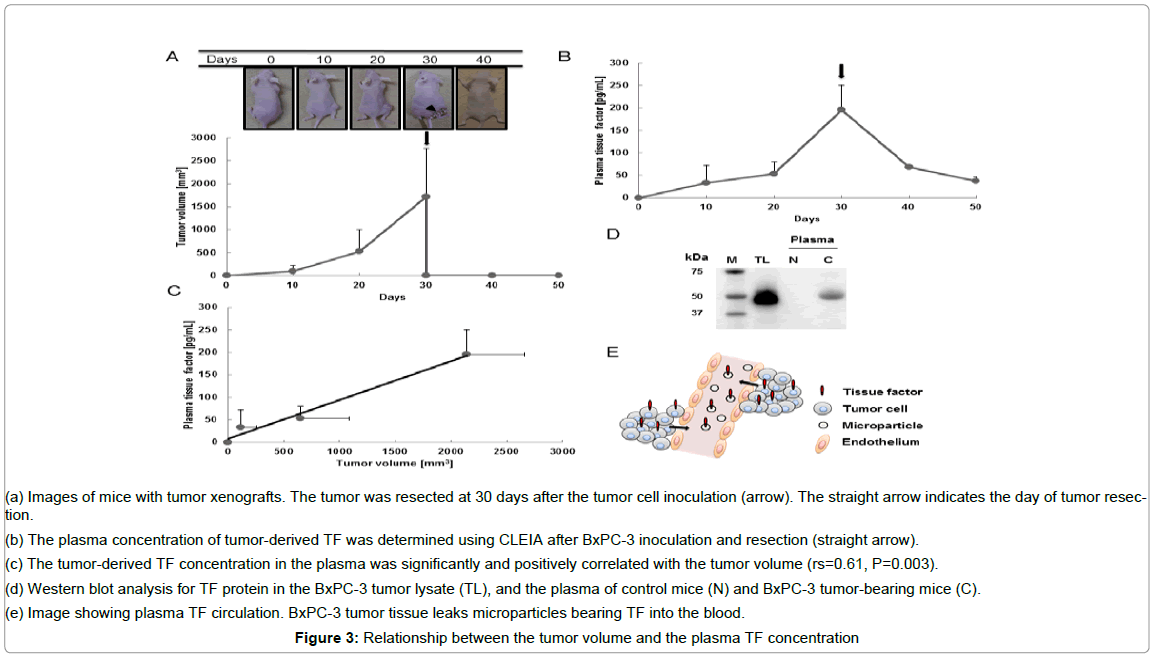
The subcutaneous tumors wereusually detectable by 10 days after tumor cell inoculation and were resected on day 30. The tumor volume was 98.1 ± 130 mm3 (mean ± SD) at 10 days, 524 ± 472 mm3 at 20 days, and 1719 ± 1046 mm3 at 30 days after tumor cell inoculation (Figure 3A). The plasma TF level was 33.1 ± 38.6 pg/mL (means ± SD) at 10 days, 53.5 ± 26.4 pg/mL at 20 days and 195 ± 54.5 pg/mL at 30 days after tumor cell inoculation (Figure 3B). The plasma TF level also decreased markedly after tumor resection: the plasma TF level was 67.5 ± 4.21 pg/mL at 10 days after resection and 37.6 ± 8.62 pg/mL at 20 days after resection, as determined using CLEIA (Figure 3B). The plasma TF level was positively correlated with the tumor volume (Figure 3C), rs = 0.61, P = 0.003. TF was detected in the tumor lysate and plasma of the tumor-bearing mice. The molecular weight of the TF detected from the tumor lysate was 47-kDa. In addition, the TF detected in the plasma of the tumor-bearing mice (C) had a molecular weight of 47-kDa (Figure 3D). Meanwhile, the TF was not detected in the plasma of normal mice (N).
Figure 3: Relationship between the tumor volume and the plasma TF concentration
(a) Images of mice with tumor xenografts. The tumor was resected at 30 days after the tumor cell inoculation (arrow). The straight arrow indicates the day of tumor resection.
(b) The plasma concentration of tumor-derived TF was determined using CLEIA after BxPC-3 inoculation and resection (straight arrow).
(c) The tumor-derived TF concentration in the plasma was significantly and positively correlated with the tumor volume (rs=0.61, P=0.003).
(d) Western blot analysis for TF protein in the BxPC-3 tumor lysate (TL), and the plasma of control mice (N) and BxPC-3 tumor-bearing mice (C).
(e) Image showing plasma TF circulation. BxPC-3 tumor tissue leaks microparticles bearing TF into the blood.
Discussion
This study clearly showed that the sensitivity of CLEIA was superior to that of the commercially available ELISA kit. In addition, CLEIA appeared to be a simple and quick method. These features suggest that CLEIA could be a major tool for protein detection systems. Recently, a relationship between the tumor-derived plasma TF concentration and blood coagulation has been reported in mouse xenograft models of human pancreatic cancer [9]. Unfortunately to date, an association between tumor-derived TF and tumor volume has not been shown. In this study, we established CLEIA as a TF detection system with a sensitivity sufficient to distinguish changes in the plasma TF concentration reflecting tiny variations in the tumor volume. Plasma TF-bearing micro particles may leak from the tumor tissue into the circulation (Figure 3E). This study clearly showed that the plasma TF level was positively and significantly correlated with the tumor volume. When the tumor was completely resected, plasma TF gradually decreased. Plasma TF-bearing microparticle could remain in the blood for several days.
Since the plasma level of tumor-derived TF was positively correlated with the tumor volume, the plasma TF concentration may be a useful tumor marker. Furthermore, CLEIA may help to elucidate the influence of plasma TF on cancer progression.
Acknowledgements
This work was supported by the National Cancer Centre Research and Development Fund (Yasuhiro Matsumura, Y.M.), and the Funding Program for World-Leading Innovative R&D in Science and Technology (FIRST Program) (Y.M.).
References
- Candido J, Hagemann T (2013) Cancer-related inflammation. J ClinImmunol 33: 79-84.
- Lipinski S,Bremer L,Lammers T, Thieme F,Schreiber S (2011) Coagulation and inflammation. Molecular insights and diagnostic implications. Hamostaseologie 31: 94-102, 104.
- Van den Berg YW, Osanto S,Reitsma PH,Versteeg HH (2012) The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 119: 924-32.
- Robert WC, Alexander WC, James NG, Samuel ZG (2006) Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Lippincott Williams & Wilkins.
- Date K,Jessica H, John G, Anthony M, Leigh AM (2013)Tumour and microparticle tissue factor expression and cancer thrombosis. Thromb Res, 131: 109-15.
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood122: 1712-1723.
- Saito Y, Hashimoto Y, Kuroda J, Yasunaga M, Koga Y, et al. (2011) The inhibition of pancreatic cancer invasion-metastasis cascade in both cellular signal and blood coagulation cascade of tissue factor by its neutralization antibody. Eur J Cancer 47: 2230-2239.
- Falzarano R,Viggiani V, Michienzi S, Longo F, Tudini S, et al. (2013) Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. TumourBiol 34: 3093-3100.
- Wang JG,Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, et al. (2012) Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 119: 554 3-52.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 16338
- [From(publication date):
December-2014 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 11591
- PDF downloads : 4747