A Multicentre Comparative Study of Radiotherapy Alone and Radiotherapy with Concurrent Followed by Sequential Temozolomide for High Grade Glioma in Bangladesh
Received: 10-Apr-2018 / Accepted Date: 20-Jul-2018 / Published Date: 26-Jul-2018
Keywords: Glasgow Coma Scale; Karnofsky Performance Score (KPS); Radiotherapy (RT); Temozolomide (TMZ)
Introduction
Primary brain tumours are diverse group of neoplasms arising from different cells of the central nervous system. Light microscopy classifies these tumours according to predominant cell type and grades them for malignancy according to standard histopathological features. Gliomas are the most common malignant primary brain tumours (80%) and constitute 32% of all brain and CNS tumours. Gliomas arise from astrocytes (astrocytoma), oligodendrocytes (oligodendroglioma) and ependymal cells (ependymoma). Astrocytoma’s account for approximately three-quarters of gliomas [1]. The term High Grade Glioma (HGG) or malignant glioma is used to describe WHO grade III and IV primary intracranial tumours. Grade III tumours include anaplastic astrocytoma, mixed anaplastic oligoastrocytoma and anaplastic oligodendroglioma. Glioblastoma multiforme (GBM) is the only Grade IV primary intracranial tumour [2]. High grade gliomas account for approximately 20% of all intracranial neoplasms and for more than 80% of gliomas of the cerebral hemispheres in adult. Of those, GBM accounts for 60 to 70%, anaplastic astrocytoma for 10 to 15%, anaplastic oligoastrocytoma and anaplastic oligodendroglioma for about 10% [3]. The median survival of patients with high-grade gliomas) is only 15 months [4].
Although high grade gliomas account for only 2% of all cancers and are one-fifth as common as lung or breast cancers, they contribute to substantial morbidity, and prognosis is poor. The 5-year survival rate for high grade gliomas (36% during 1999 to 2005) is the sixth lowest among all type of cancers. The 5-year survival rates vary substantially by histological subtypes, 79.1% for oligodendroglioma, 27.4% for anaplastic astrocytoma and 4.5% for glioblastoma multiforme [5,6]. Surgery ranges from diagnostic biopsy to debulking to gross total resection 6. Biopsies are minimally invasive, well tolerated and suitable for lesions of any site or size. Biopsy is usually considered when risk of resection outweighs the benefit [7]. Debulking surgery is beneficial in reducing the tumour load, lowering ICP and providing a more representative histological sample [6]. Radiotherapy (RT) has been the mainstay of adjuvant therapy for HGGs since multiple studies from the 1970s showed a survival benefit [6]. However, addition of chemotherapy with Temozolomide (TMZ) with RT has shown better outcomes in several studies. A randomized control study on 573 patients of glioblastoma multiforme from 85 centres in Europe was conducted with RT (fractionated focal irradiation in daily fractions of 2 Gy given 5 days per week for 6 weeks, for a total of 60 Gy) with or without TMZ, concomitant 75 mg/m2 of body surface area daily from the first to the last day of RT, followed by six cycles of adjuvant TMZ 150 to 200 mg/m2 for 5 days during each 28-day cycle). 84% of those patients underwent debulking surgery. The study revealed median survival 14.6 months with RT plus TMZ and 12.1 months with RT alone. The hazard ratio for death was 0.63 in the radiotherapy plus temozolomide group (p<0.001). Two-year survival was 26.5% with RT plus TMZ and 10.4% with RT alone [2]. Concomitant RT plus TMZ resulted in grade 3 or 4 hematologic toxic effects in 7% patients 2. Another study revealed median and 1-year survival 13.6 months and 48% in RT plus TMZ, in compared to 8.9 months and 15.7% in RT alone groups [8]. A study was conducted on 46 glioblastoma patients in Germany who received RT with TMZ. A local progression was detected in 70% patients. The median survival time amounted to 13.6 months resulting in one-year and two-year survival probabilities of 48% and 8%, respectively [9]. Our study was aimed to provide a comparison between the proportion of disease progression, adverse effect in GBM patients treated with radiotherapy with concurrent and sequential Temozolomide to those treated with radiotherapy alone. Also, we aimed to investigate patient’s short-term response in the time interval of this study and calculate Progression Free Survival (PFS) and overall survival (OS).
Materials And Methods
This comparative study was carried out in the department of Radiation Oncology of NICRH, Dhaka & Square Hospitals Limited, Dhaka from April 2014 to January 2015. The study was approved by the Ethical Review Committee of NICRH and informed consent was taken from each patient before their enrolment in the study. Total study population was 60. They were randomly divided into two treatment groups, 30 patients in each. Group A patients received Radiotherapy (RT) plus concomitant, followed by sequential Temozolomide (TMZ) according to standard dose and schedule Group B patients were treated with RT alone. All patients received RT to limited fields once daily at 2 Gy per fraction, 5 days a week, for a total of 60 Gy, and the dose were prescribed according to the guidelines of the International Commission of the Radiological Units. The patients were treated with thermoplastic immobilization masks to ensure adequate immobilization during therapy and reproducibility. The treatment volumes for both the initial volume and the boost volume was based on preoperative computed tomography (CT) and/or magnetic resonance imaging (MRI) scans. For the first 46 Gy, the initial treatment volume was determined by the volume of contrast-enhancing tumour and surrounding edema calculated from CT and/or MRI scan plus a 2-cm margin or 2.5-cm margin if no surrounding edema present. After 46 Gy, the treatment volume was reduced to the contrast-enhancing tumour (without edema) on the preoperative CT and/or MRI scan plus a 2.5-cm margin to a total dose of 60 Gy. Radiotherapy planning for all patients were done using three-dimensional techniques. Patients assigned to the combined-modality group received TMZ (75 mg/m2 for 7 days a week), concomitantly with RT, 1 hour before irradiation and in the morning on days without RT. Four weeks after RT, patients received six cycles of adjuvant TMZ. 150 mg/m2 of TMZ on days 1 through 5 and 15 to 19 every 28 days), aiming at dose intensification. Prophylactic antiemetic were used routinely. Anticonvulsants and corticosteroids were administered as needed.
Patients were assessed weekly during RT for toxicity. CBC count was performed weekly during treatment, and blood chemistry was performed monthly. During adjuvant TMZ cycles, neurologic examinations, serum chemistry, anticonvulsant levels, and toxicity evaluations were performed at each TMZ cycle and at every follow-up appointment in the RT alone group at every 2 months during study period of one year. 3 months during the second year after study entry). CT or MRI scans with or without contrast was performed before the first adjuvant treatment cycle and then every 2 months. Progression free survival and overall survival were assessed.
Patients were managed symptomatically with antibiotics, analgesics, steroids, Antihistamines, anti-emetics, vitamins, IV fluids and blood transfusion on need basis. Response was evaluated as per guideline of WHO18 and RECIST20. To declare response follow up endoscopy and biopsy were done 6 weeks after completion of treatment (either RT or CT-RT).
Finally, the data analysis was done using standard statistical procedures. Statistical Products and Service Solutions (SPSS) version 20 was used whenever required and to crosscheck the results. Qualitative and quantitative data were analysed with chi-square and unpaired T tests respectively. Associations were expressed in terms of relative risk and considered statistically significant if p-value was <0.05.
Sample size
Sample size for comparative studies comparing proportions (WHO 2001)
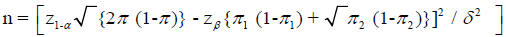
π1 – expected proportion in one group
π2 – expected proportion in another group
π=(π1 + π2) / 2
δ=clinically significant difference
Conventionally,
Type I error, α=0.05
Power, 1 - β=0.8
δ=0.1
For disease progression
• π1=0.54, π2=0.36 17
• n=116 for each group
For toxicity
• π1=0.15, π2=0.05 16
• n=138 for each group
Sample size in previous similar studies varied between 46 and 563. Due to time restrain, sixty (60) patients were selected for this study, thirty (30) for each group.
Sampling technique
Non-randomized convenience sampling.
Selection criteria
Newly diagnosed patients of both sexes and age ≥ 18 years with histologically confirmed high grade glioma were selected for the study. Patients with Karnofsky performance score <60, inadequate bone marrow reserve (Haemoglobin <10 gram/dL, total leukocyte count <4,000/cu mm, neutrophil count <1,500/cu mm or platelet count <100,000/cu mm), impaired renal function (Serum creatinine >1.5 mg/dL), abnormal liver function (serum bilirubin >1.5 times, SGPT >2.5 times or alkaline phosphatase >2 times of upper limit), serious co-morbidity (e.g. severe infection, heart failure, metabolic encephalopathy) and unwilling to give consent were excluded from the study.
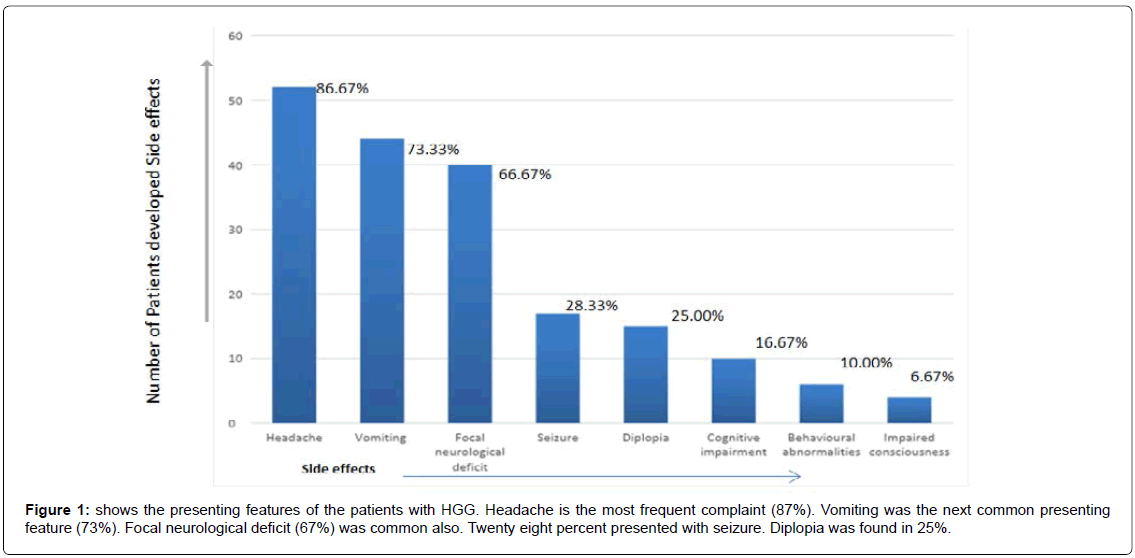
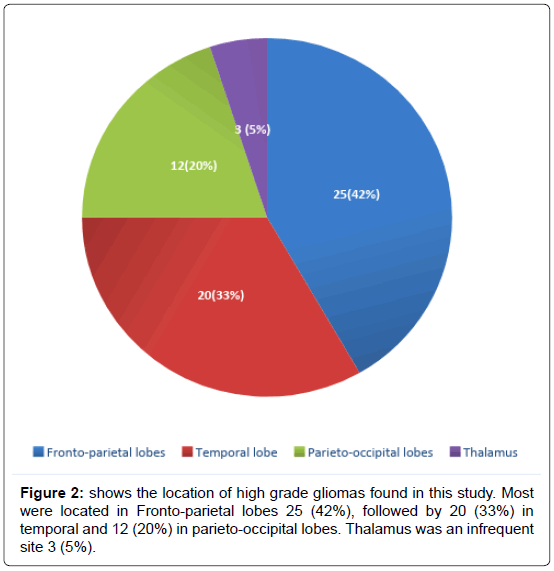
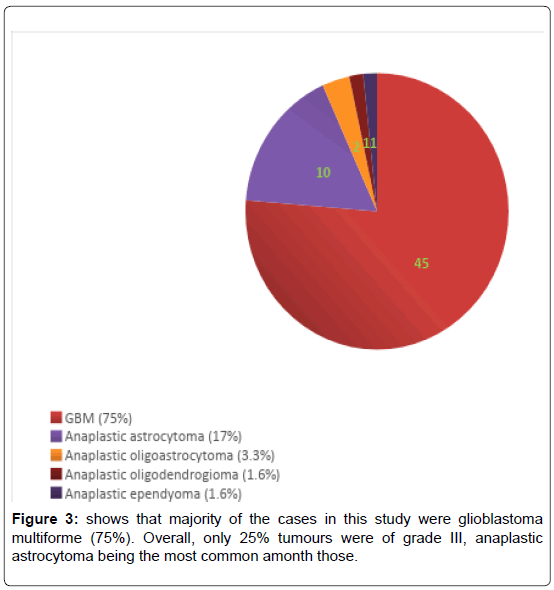
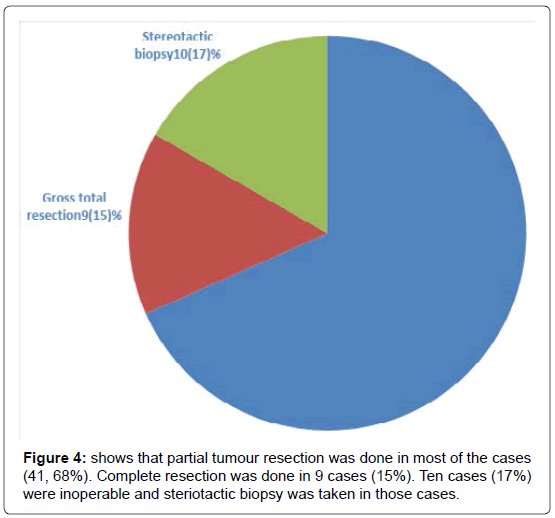
We have included (Table 1) total sixty patients of high grade glioma in the study and among them 38 (63%) were males and 22 (37%) were females. The mean age of Group A was 59.0 (SD ± 12.2) and Group B was 59.0 (SD ± 10.8). In Group A 18 (60%) patients were male, in Group B the number of male were 20 (67%). Majority of the patients were from rural areas, Muslims, married and having monthly income of Taka 10,000 or less (Table 2). There was no statistically significant difference between the two groups when we compared age, sex, Karnofsky performance scale, Glasgow coma scale, haemoglobin level, total leukocyte count and platelet count (Table 3). Headache, vomiting and focal neurological deficit were respectively most frequent complaint (Figure 1). Most of the high-grade gliomas were in fronto-parietal lobes 42%, followed by 33% in temporal and 20% in parieto-occipital lobes. Thalamus was an infrequent site 5% (Figure 2). Histopathological diagnosis showed majority of the cases in this study were glioblastoma multiforme (75%) (Figure 3). Partial tumour resection was done in 68% of the cases. Complete resection was done in 15% cases and 17% were inoperable and stereotactic biopsy was taken in those cases (Figure 4).
| Age (years) | Male | Female | Total |
|---|---|---|---|
| 18 – 30 | 1 (2.6) | 0 (0.0) | 1 (1.7) |
| 31–40 | 2 (5.3) | 2 (9.1) | 4 (6.7) |
| 41–50 | 7 (18.4) | 5 (22.7) | 12 (20) |
| 51–60 | 11 | 5 | 16 |
| (28.9) | (22.7) | (26.6) | |
| 61–70 | 14 (36.9) | 8 (36.4) | 22 (36.6) |
| 71–80 | 2 (5.3) | 2 (9.1) | 4 (6.7) |
| >80 | 1 (2.6) | 0 (0) | 1 (1.7) |
| Total | 38 (100) | 22 (100) | 60 (100) |
| Mean (years) | 59.1 ± 11.3 | 59.2 ± 11.8 | 59.1 ± 11.4 |
| Range (years) | 30–85 | 35–80 | 35–85 |
Table 1: Age and sex distribution, Total sixty patients of high grade glioma were included in the study. Thirty-eight (63%) were males and twenty-two (37%) were females. Majority of cases aged more than 50 years (72%), in both sexes (74 and 68% among males and females respectively). (Within the Table, Percentages are mentioned within parenthesis).
| Variable | Data | Frequency | Percentage |
|---|---|---|---|
| Residence | Rural | 36 | 60.0 |
| Urban | 24 | 40.0 | |
| Marital status | Single | 0 | 0.0 |
| Married | 41 | 68.4 | |
| Widowed | 17 | 28.3 | |
| Divorced | 2 | 3.3 | |
| Religion | Islam | 52 | 86.6 |
| Hinduism | 7 | 11.7 | |
| Christianity | 1 | 1.7 | |
| Monthly income (Taka) | <5,000 | 17 | 28.3 |
| 5,000–10,000 | 28 | 46.7 | |
| >10,000 | 15 | 25.0 |
Table 2: Demographic variables , It shows some basic demographic variables of the patients in this study. Majority of the patients were from rural areas, Muslims, married and having monthly income of Taka 10,000 or less.
| Variables | Group A RT plus TMZ |
Group B RT |
p value | |
|---|---|---|---|---|
| Age (years) | 59.0 ± 12.2 | 59.0 ± 10.8 | 0.30* | |
| Sex | Male | 18 | 20 | 0.78** |
| Female | 12 | 10 | ||
| KPS (>60) | ≥ 80 | 28 | 27 | ** |
| <80 | 2 | 3 | ||
| GCS | 13-5 | 28 | 29 | ** |
| 3-2 | 2 | 1 | ||
| Haemoglobin (gm/dL) | 11.4 ± 1.5 | 11.5 ± 1.9 | 0.63* | |
| Total leukocyte count (1000 per cu mm) |
7.5 ± 1.5 | 7.2 ± 1.5 | 0.80* | |
| Platelet count (100,000 per cu mm) |
2.1 ± 0.6 | 2.0 ± 0.5 | 0.78* | |
Table 3: Comparison of baseline variables between the two groups, it shows some important baseline variables in the study. There was no statistically significant difference between the two groups in respect of age, sex, Karnofsky performance scale, Glasgow coma scale, haemoglobin level, total leukocyte count and platelet count. (Within the Table, Percentages are mentioned within parenthesis).
Higher proportion of patients was found progression free in Group A patients than Group B, (73% versus 43%) which was statistically significant (p-value 0.04) (Table 4). Regarding hematological toxic effects, Anaemia (40% versus 33%), leucopenia (60% versus 33%) and thrombocytopenia (53% versus 27%) occurred in higher proportion of Group A, but that was not statistically significant (p>0.05) (Table 5). Non-hematological toxic effects, such as nausea, vomiting, headache, constipation and skin reaction were more frequent in Group A and Otitis externa occurred more in Group B. But none of these differences was statistically significant (Table 6).
| Outcome | Group A RT plus TMZ |
Group B RT |
Odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| (n1=30) | (n2=30) | |||||
| Progression free survival | Yes | 22 (73%) |
13 (43%) |
3.60 | 1.22 – 10.64 | 0.04* |
| No | 8 (27%) |
17 (57%) |
||||
Table 4: Disease Progression, It shows the comparison between the progression free survival of 60 patients at the end of 8 weeks. Higher proportion of patients was found progression free in patients receiving radiotherapy plus temozolomide than those receiving radiotherapy alone (73% versus 43%) which was statistically significant (p-value 0.04). (Within the Table, Percentages are mentioned within parenthesis).
| Toxicity | Group A RT plus TMZ (n1=30) |
Group A RT (n2=30) |
Relative risk | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Haemoglobin (gm/dL) |
<10 | 12 (40) |
10 (33) |
1.33 | 0.47 – 3.82 | 0.79* |
| ≥ 10 | 18 (60) |
20 (60) |
||||
| Total leukocyte count (per cu mm) |
< 4,000 | 18 (60) |
10 (33) |
3.00 | 1.05 – 8.60 | 0.07* |
| ≥ 4,000 | 12 (40) |
20 (67) |
||||
| Platelet count (per cu mm) |
<100,000 | 16 (53) |
8 (27) |
3.14 | 1.07–9.27 | 0.06* |
| ≥ 100,000 | 14 (47) |
22 (73) |
||||
Table 5: Haematological toxicity, It compares the proportion of anaemia, leucopenia and thrombocytopenia between the two groups. Anaemia, leucopenia and thrombocytopenia occurred in higher proportion of patients in group A, but that was not statistically significant (p > 0.05). (Within the Table, Percentages are mentioned within parenthesis).
| Toxicity | Group A RT plus TMZ (n1=30) |
Group A RT (n2=30) |
Relative risk | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Nausea | Yes | 28(45) | 23(22) | 4.26 | 0.81–22.53 | 0.15* |
| No | 2(55) | 7(78) | ||||
| Vomiting | Yes | 26(48) | 17(22) | 3.76 | 1.04–13.65 | 0.07* |
| No | 4(52) | 13(78) | ||||
| Headache | Yes | 24(21) | 22(15) | 1.46 | 0.44–4.86 | 0.76* |
| No | 6(79) | 8(85) | ||||
| Constipation | Yes | 15 (50) | 13(43) | 1.31 | 0.47–3.62 | 0.80* |
| No | 15 (50) | 17(57) | ||||
| Alopecia | Yes | 18 (60) | 2(7) | 14.00 | 2.81–157.15 | <0.01* |
| No | 12 (40) | 28(93) | ||||
| Skin reaction | Yes | 3(10) | 0(0) | 7.76 | 0.38–4.86 | 0.24* |
| No | 27(90) | 30(100) | ||||
| Otitis externa | Yes | 2(7) | 4(14) | 0.46 | 0.08–2.75 | 0.67* |
| No | 28(93) | 26(86) | ||||
Table 6: Non-haematological toxicity, It compares the proportions of nonhaematological toxicities between the two groups. Nausea, vomiting, headache, constipation and skin reaction were more common in radiotherapy plus temozolomide group, whereas otitis externa was more frequent in radiotherapy alone group. However, none of these associations were statistically significant (p>0.05). The only statistically significant difference was found for alopecia, which occurred in higher proportion in radiotherapy plus temozolomide group (p<0.01). (Within the Table, Percentages are mentioned within parenthesis).
Discussion
A total 60 patients of newly diagnosed high-grade glioma were enrolled for the study and randomly divided into two treatment groups. Group A patients received Radiotherapy (RT) plus concomitant, followed by sequential Temozolomide (TMZ) according to standard dose and schedule Group B patients were treated with RT alone. All patients received RT to limited fields once daily at 2 Gy per fraction, 5 days a week, for a total of 60 Gy. Patients assigned to the combinedmodality group (Group B) received TMZ (75 mg/m2 for 7 days a week), concomitantly with RT, 1 hour before irradiation and in the morning on days without RT. Four weeks after RT, patients received six cycles of adjuvant TMZ. 150 mg/m2 of TMZ on days 1 through 5 and 15 to 19 every 28 days), aiming at dose intensification.
Mean age of the participants was 59.1 ± 11.4 years, with the youngest and the eldest ones were 30 and 85-year-old, respectively. The age distribution was similar to previous studies on RT with or without TMZ [2,9].
Thirty-eight (63%) patients were male and 22 (37%) were females. The male-female ratio was 1.72, which was similar to that of most of the previous researches [10,11]. However, the ratio was much lower than that was found in India [12].
Majority of the patients were from rural areas, Muslims, married and retired with monthly income of Taka 10,000 or less, like that of India, except the religion, as most of the Bangladeshi people are Muslims, whereas, most of the Indian people are Hindus.
The Karnofsky Performance Score (KPS) was 80 or more in 55 patients. Glasgow Coma Scale (GCS) score was 13 or more in 57 patients. Mean hemoglobin level, total leukocyte count and platelet count were 11.4 ± 1.6 gram per deciliter, 7.4 ± 1.5 thousand per mg/ m2 and 2.1 ± 0.5 lacs per cubic millimeter, respectively. These baseline variables were similar to previous studies 2,9. There was no statistically significant difference between the two treatment groups regarding age and sex distribution, KPS, GCS and blood counts.
Headache is the most frequent complaint (87%). Vomiting was the next common presenting feature (73%). Focal neurological deficit (67%) was common also. Twenty-eight% presented with seizure. Diplopia was found in 25%. Similar findings were found in studies conducted in India [12].
Most of the HGGs in this study were located in Fronto-parietal lobes 25 (42%), followed by 20 (33%) in temporal and 12 (20%) in parieto-occipital lobes. Thalamus was an infrequent site 3 (5%).
Regarding histopathological diagnosis, majority of the cases in this study were glioblastoma multiforme (75%). Overall, only 25% tumours were of grade III, anaplastic astrocytoma being the most common among those, a similar finding with previous studies [6-9].
Regarding surgical management, partial tumour resection was done in most of the cases (41, 68%). Complete resection was done in 9 cases (15%). Ten cases (17%) were inoperable and stereotactic biopsy was taken in those cases. The proportion of complete resection was quite lower than that of patients in previous studies (50 to 60%) 2,9. Most of those studies were conducted in Europe and North America. So an earlier diagnosis and superiority in surgical techniques of these regions might explain this discrepancy.
Patients were followed up after 8 weeks of treatment with RT for a total of 60 Gy (to limited fields once daily at 2 Gy per fraction, 5 days a week) with or without TMZ (75 mg/m2). Tumour progression was found in 8 patients (27%) in RT plus TMZ group and 17 patients (57%) in RT alone group. Higher proportion of patients was found progression free in patients receiving RT plus TMZ than those RT alone (73 versus 43%) which was statistically significant (p-value 0.04). The results we have found is similar to that reported by Stupp et al. and Niewald et al.
Regarding hematological toxic effects, Anaemia (40 versus 33%), leucopenia (60 versus 33%) and thrombocytopenia (53 versus 27%) occurred in higher proportion of patients in RT plus TMZ group, but that was not statistically significant (p>0.05). Niewald et al. found similar results.
Non-hematological toxic effects, such as nausea, vomiting, headache, constipation and skin reaction were more frequent in RT plus TMZ group and Otitis externa occurred more in RT alone group. But none of these differences was statistically significant, like that in studies of Niewald et al. and Stupp et al. However, alopecia occurred in significantly higher proportion of patients of RT plus TMZ group. Niewald et al. revealed similar result.
Conclusion
High grade gliomas bear very poor prognosis despite advent of newer treatment options. This study showed better progression free survival in high grade glioma patients receiving radiotherapy plus Temozolomide than those treated with radiotherapy alone. There was no significant difference in toxic effects between these two treatment options, except higher incidence of alopecia among temozolomide treated patients. So, addition of Temozolomide with radiotherapy may improve the overall survival and prognosis in high grade glioma patients without significant risk of toxic effects. A similar study with larger sample and longer follow-up is highly recommended for developing country like Bangladesh.
References
- Larjavaara S, Mäntylä R, Salminen T (2007) Incidence of gliomas by anatomic location. Neuro Oncol 9: 319-325.
- Stupp R, Mason WP, van den Bent MJ (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
- Price S, Narayanan V, Patel, K (2012) High grade gliomas: pathogenesis, management and prognosis. Advances in Clinical Neuroscience and Rehabilitation 12: 24-29.
- Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJB (2014) Health-related quality of life in high-grade glioma patients. Chinese Journal of Cancer 33: 40-45.
- Daroff RB, Bradley WG (2012) Bradley's neurology in clinical practice. Elsevier.
- Amundson EW, McGirt MJ, Olivi A (2005) A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg; 102: 565-570.
- Athanassiou H, Synodinou M, Maragoudakis E (2005) Randomized Phase II Study of Temozolomide and Radiotherapy Compared With Radiotherapy Alone in Newly Diagnosed Glioblastoma Multiforme. Journal of Clinical Oncology 23: 2372-2377.
- Niewald M, Berdel C, Fleckenstein J, Licht N, Ketter R, et al. (2011) Toxicity after radiochemotherapy for glioblastoma using temozolomide--a retrospective evaluation. Radiat Oncol 6: 141.
- McKinney P (2004) Brain Tumours: Incidence Survival, And Aetiology. Journal of Neurology, Neurosurgery, and Psychiatry 75: ii12 - ii7.
- Ropper AH, Maurice V, Martin AS, Raymond D (2009) Adams and Victor's Principles of Neurology. 9 ed. New York, : McGraw Hill Medical.
- Krishnatreya M, Kataki AC, Sharma JD, Bhattacharyya M, Nandy P, et al. (2014) Brief descriptive epidemiology of primary malignant brain tumors from North-East India. Asian Pac J Cancer Prev 15: 9871-9873.
- Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S (2005) A population-based description of glioblastoma multiforme in Los Angeles County, 1974-1999. Cancer 104: 2798-2806.
Citation: Sharmeen F, Chowdhury Q, Reza MS, Wazib A, Saha CK, et al. (2018) A Multicentre Comparative Study of Radiotherapy Alone and Radiotherapy with Concurrent Followed by Sequential Temozolomide for High Grade Glioma in Bangladesh. Adv Cancer Prev 3: 127.
Copyright: © 2018 Sharmeen F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Usage
- Total views: 4374
- [From(publication date): 0-2018 - Mar 09, 2025]
- Breakdown by view type
- HTML page views: 3577
- PDF downloads: 797