A Multi-Center Pilot Study to Evaluate the Efficacy and Safety of NonDimethyl Sulfoxide (Non-Dmso) Viable Umbilical Cord Graft on Diabetic Foot Ulcers
DOI: 10.4172/2329-910X.1000320
Abstract
Diabetic Foot Ulcers (DFUs) are one of the main reasons for diabetes-related hospitalizations, all the while creating an economic burden on the healthcare system and considerably impairing quality of life. In cases where a wound fails to decrease in size by 50% within 4 weeks with standard-of-care, it may be appropriate to initiate advanced levels of care to attempt to close the wound and limit complications. Use of amnion/chorion-based skin substitutes that have been cryopreserved and contain viable cells have been shown to achieve full wound closure with less treatments and in less time. Currently, the standard for cryopreservation of mesenchymal stem cells (MSCs) is done with dimethyl sulfoxide (DMSO), which is easily permeable and able to protect MSCs from cryo-injuries. However, DMSO has been shown to have deleterious effects on the viability of cells and tissue, and has adverse effects in humans. Therefore, there is a growing effort to develop DMSO-free cryoprotectants. This pilot study is being presented to study the effects and safety of non-dimethyl sulfoxide (NonDMSO) viable umbilical cord graft (UCG) on DFUs. 25 patients were screened for the study, with 15 patients completing the trial. 6/15 (40%) had complete closure prior to or at the study endpoint. Of the patients whose ulcers did not close by Week-12 (9/15 patients, 60%), eight patients had a 50% or greater improvement by Week-12 (88.8%), and five of those ulcers (55.5%) had a closure rate 90% or greater by week 12. Among the entire cohort, fourteen patients (93.3%) had 50% or greater wound reduction by Week-12, and eleven patients (73.3%) patients had 90% or greater wound reduction by Week-12. Only one patient had 2 applications of graft; the remaining 14 had one application only. Further studies need to be designed to compare DMSO vs. Non-DMSO UCG effectiveness in slow-to-heal DFUs. However, based on our findings, non-DMSO viable umbilical cord graft is an effective way to treat challenging DFUs
Keywords: Non-dimethyl sulfoxide; Diabetic foot ulcers; Umbilical cord graf
Introduction
Diabetes mellitus has become a global epidemic, with approximately 422 million people affected worldwide, including 29 million people in the United States [1,2]. Foot ulcers are one of the main reasons for diabetes-related hospitalizations while creating an economic burden on the healthcare system and considerably impairing quality of life [3,4]. Approximately one-third of diabetes-related costs have been linked to the treatment of foot ulcers [5]. Patients with diabetes have up to a 25% lifetime risk of developing a diabetic foot ulcer (DFU) [6]. Currently, the standard of care (SOC) for initial treatment of DFUs is debridement, offloading, tight glycemic control and appropriate antimicrobial management and/or imaging when needed [7]. A meta-analysis of patients studied in controlled trials demonstrated, on average, healing rates of 31% at 20 weeks with SOC [8]. A substantial portion of these wounds will become infected over time, resulting in lower extremity minor and major amputation [9]. In cases where a wound fails to decrease in size by 50% within 4 weeks with SOC, advanced levels of care may be initiated to attempt to close the wound and limit these complications [10]. These may include topical platelet-derived growth factor (PDGF), hyperbaric oxygen therapy (HBOT), and cellular and/or tissue-based products (CTPs) [11-17].
Use of amnion/chorion-based skin substitutes that have been cryopreserved and contain viable cells have been shown to achieve full wound closure with less treatments and in less time [18]. Currently, the standard for cryopreservation of mesenchymal stem cells (MSCs) is done with dimethyl sulfoxide (DMSO), which is easily permeable and able to protect MSCs from cryo-injuries [19]. However, the use of dimethyl sulfoxide (DMSO) in cryopreservation has been shown to have deleterious effects on the viability of cells and tissue [20-23]. Data from the European Cooperative Group for Bone Marrow Transplantation (EBMT) centers show roughly one in 70 transplants experience DMSO-related complications, with most cases being cardiovascular and respiratory in nature [24]. Even after rinsing, trace amounts of DMSO in the product remained a high risk to trigger adverse reactions in bone marrow transplant patients [25]. Additionally, DMSO has been suggested to be associated with abnormal gene expression and differentiation of MSCs in mice [18]. Therefore, there is a growing concern within the medical community to investigate and develop DMSO-free cryoprotectants.
This pilot study is being presented to investigate the effects of non- DMSO viable umbilical cord graft (UCG) on DFUs. The product being studied is non-DMSO cryopreserved placental umbilical cord allograft that is recovered from healthy mothers who have undergone Cesarean-section delivery. It retains the natural properties of placental tissue including nutrient-rich growth factors, cytokines, endogenous cells, and Wharton’s Jelly [26,27]. Placental tissue acts as an immune-privileged protective barrier during fetal development [26]. The non-DMSO UCG is applied as an anatomical barrier that helps provide mechanical protection while retaining endogenous growth factors (Figure 1) [26-28]. The proprietary process preserves the natural properties of placental umbilical cord tissue, maintaining inherent levels of key extracellular matrix molecules, including proteins, carbohydrates, growth factors, and cytokines. This investigation will evaluate the safety and efficacy of non-DMSO UCGs, as well as compare the study product to the outcomes of DMSO-preserved umbilical cord grafts, other human cells, tissues, and cellular and tissuebased products (HCT/Ps) and standard-of-care treatments currently being used for the same condition.
Methods
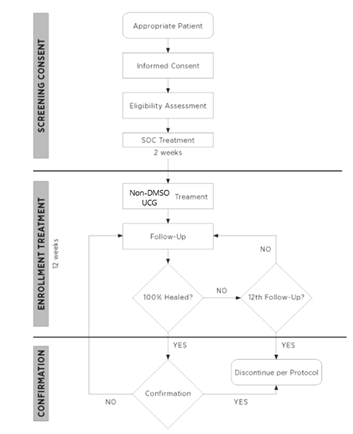
This study was submitted and approved by the Advarra Institutional Review Board (#00045210). All patients were treated and assessed by the authors in an outpatient setting. Informed consent was obtained from all patients enrolled in the trial. The total length of the pilot study was 14 weeks. Patients were selected to receive non-DMSO UCG as a treatment for their DFU, based on qualifications of the inclusion/exclusion criteria in Table 1. The DFU was assessed clinically, photographed, and measured via planimetric tracing at Screening Visit (Day 14 ± 3 days). A 2-week run in period was allotted, and all the wounds were treated with SOC alone. This included sharp surgical debridement (at screening, effectively Day 14), a saline-gel and gauze dressing which was changed daily by the patient, and offloading with a removable cast walking boot. At Treatment Visit (Day 0 ± 3 days), the wound was assessed again. If there was >30% improvement with SOC alone, the patient would not qualify for the study, as this would not be deemed difficult to heal, and not typically require use of an advanced biologic graft. If there was less than a 30% improvement in wound size, the patient qualified for application of non-DMSO UCG. The ulcer was prepared prior to application of non-DMSO UCG with sharp surgical debridement, until a healthy, bleeding wound bed was created. The non-DMSO UCG was thawed in accordance with manufacturing recommendations and secured to the wound as per the surgeon’s preference: sutures, staples, or a combination of these methods. Saline gel was applied to the outside of the graft to prevent moisture loss, and a non-adherent contact layer and multilayer compression bandage above the graft, which was left intact until the next follow up visit. Thereafter, weekly office assessments were made for a maximum of an additional 11 weeks (12 weeks in total). CAM boot compliance was assessed weekly via questionnaire. A diagram of the study progression is presented in Figure 2. If deemed appropriate by the investigator, additional applications of non-DMSO UCG were allowed to be applied at subsequent visits. The reapplication of non-DMSO UCG would follow the investigator’s clinical judgment, as would be if the patient was not enrolled in a study. Some limited examples/reasons for re-application are: improper graft adherence (slippage), seroma formation, stalled wound, or desire for enhanced tissue growth. At the end of a maximum of 12 study weeks, data on percentage complete closure and rate of closure will be compiled. As a secondary endpoint, a report on adverse events (AEs) and/ or serious adverse events (SAEs) for the duration of the study was also recorded. Patients were actively monitored during the trial for treatment related adverse events (e.g., infections, cellulitis, dermatitis, osteomyelitis, etc). All treatment related adverse events were documented in the subjects’ research record and classified based on the severity of the event (mild, moderate, severe, life threatening or death related to adverse event) and whether or not the event is, in the opinion of the treating Investigator, related to Non-DMSO UCG.
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| 1. 18 years of age or older | 1. Suspected or confirmed signs of infection of the study ulcer/limb |
| 2. Type I or II diabetes mellitus | 2. Sensitivity to study material |
| 3. Glycosylated hemoglobin (HbA1c)<12% within 3 months of Non-DMSO UCG application | 3. Pregnancy |
| 4. Adequate vascular perfusion of affected limb as determined by ankle-brachial index (0.9 or better) | 4. Receiving medication/treatment known to affect wound healing within 30 days of treatment visit |
| 5. Willing and able to maintain required off-loading of affected limb and perform necessary dressing changes | 5. Excessive lymphedema that could interfere with wound healing |
| 6. DFU is full thickness (Wagner Grade I or II) | 6. Charcot foot with a bony deformity |
| 7. DFU is >1 square centimeter and <12 square centimeter | 7. Chopart’s amputation |
| 8. Duration of DFU is at least 30 days at the time of screening | 8. History of bone cancer of the affected limb |
| 9. Treatment with cellular/tissue-based product or platelet-derived growth factor (PDGF) within 30 days of treatment visit | |
| 10. HBOT within 3 days of treatment visit | |
| 11. Size of ulcer following debridement improved by greater than 30% during the Run-In Phase |
Table 1: Inclusion-Exclusion Criteria.
Data analyses for this paper were generated using R software, version 4.0.5 [29]. Continuous variables were summarized by mean, standard deviation, and range as appropriate. Normality was assessed using the Shapiro Wilk normality test. A paired t-test was used to assess the wound size difference over time post the graft application. Statistical significance was defined at the 5% (p ≤ .05) level.
Results
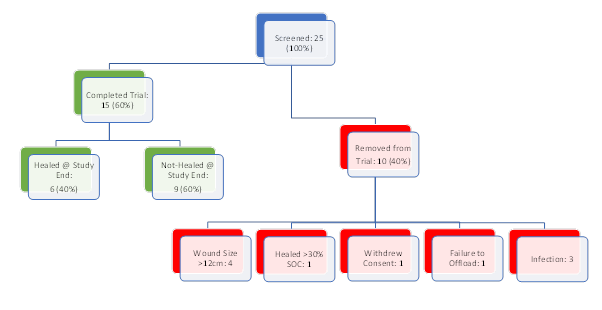
25 patients were screened for the trial from September 2020 to November 2020. Four patients failed screening due to sizes larger than 12 cm2 and one patient was removed due to the improvement greater than 30% with SOC. Among the rest, one patient withdrew consent prior to application of the graft, and one patient was removed due to inability to offload with a CAM boot properly. The remaining three patients were removed for infection, with one of those patients requiring a transmetatarsal amputation. None of the adverse events were deemed to be directly linked to the tissue graft. Fifteen patients (20% female with n=3) completed the study. A summary of enrollment is presented in Figure 3.
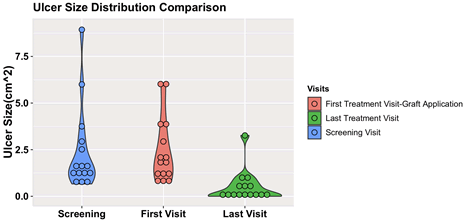
All patients had diabetic pedal ulcers. The wounds were planimetrically traced and photographed. They were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA) for wound surface area over time by setting a scale based off a known object (i.e., ruler) in the image, and tracing the outer edges of the wound. The mean ulcer size of the eligible patients at the screening visit was 2.41 cm2 (SD=2.28 cm2). A 2 week run in phase was performed, where SOC alone was used to manage the DFUs. With comparison to the mean ulcer size at screening versus the first treatment visit, there were no differences within the healed group, the non-healed group or the entire cohort respectively (healed, P=.53; nonhealed, P=.93; entire cohort, P=.58). The analysis after the run-in phase indicates that all the wounds studied in the trial were stalled wounds that would otherwise not likely heal with SOC. The distribution of the ulcer size at the screening and the first treatment visits can be visualized in Figure 4. In the post hoc analysis, there was a statistically significant difference of the mean ulcer size between the healed and the not-healed group at the screening visit and first treatment visit (Table 2). This logically implies that the larger wounds may take a longer time to close.
| All (n=15) | Healed (n=6) | Not-Healed (n=9) | P-value | |
|---|---|---|---|---|
| Ulcer Size -Screening (mean cm2, SD) | 2.41 ± 2.28 | 1.44 ± 1.15 | 3.05 ± 2.67 | 0.05 |
| Ulcer Size -Visit 1 (mean cm2, SD) | 2.44 ± 1.76 | 1.51 ± 1.14 | 3.06 ± 1.87 | 0.03 |
Table 2: Ulcer summary at the screening and the first visit (graft application).
Of the 15 patients treated with Non-DMSO UCG, only one patient (1/15, 6.7%) was treated with a second application graft at Visit #9 due to a stalled wound. The remaining 14 patients were treated with one application of the umbilical tissue graft.
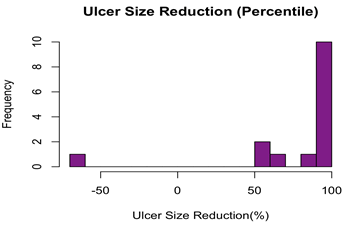
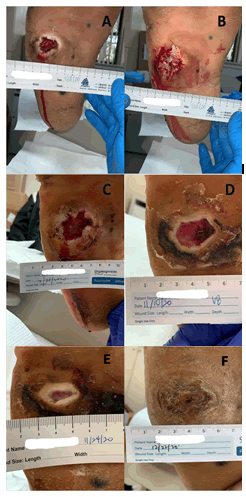
Complete ulcer closure within 12 weeks was observed in six patients (40.0%), assessed by the treating physicians based on the criteria outlined. In contrast, nine patients (60.0%) had ulcers which failed to close by Week 12 of the trial. Of the nine patients whose ulcers did not close by week 12, eight patients (88.8%) had the ulcers closed 50% or greater by week 12, and five of those ulcers (55.5%) had closed 90% or greater by week 12. Among the entire cohort, fourteen patients (93.3%) had 50% or greater wound reduction by week 12, and eleven patients (73.3%) patients had 90% or greater wound reduction by week 12. Figure 5 shows the ulcer size reduction by week 12. A clinical example is shown in Figures 6A-6F.
Regarding the 50% closure by post graft application Week 4 criterion, ten patients had an ulcer size reduction greater than 50% (66.7%) and five patients (33.3%) had an ulcer size reduction less than 50% (Table 3). Among those patients with at least 50% ulcer size reduction at Week 4, 50% (n=5) of them were completely healed by Week 12. In comparison, among the five patients with less than 50% ulcer size reduction by Week 4, only one patient (25%) with the ulcer was healed within the same timeframe. However, the patients who had a 50% reduction in ulcer size by 4 weeks did not have a statistically significant greater proportion of ulcers healed For patients whose ulcer healed within the study period, the mean time to wound closure was 8.8 weeks (range, 4–12 weeks). The effective mean weekly healing rate was 13.6%, 5.0%, and 8.6% for patients whose ulcers healed, not-healed and the entire cohort, respectively. The difference in mean percent healing rates between patients whose ulcers healed and did not heal was significant (P=.001). The mean absolute weekly reduction in ulcer size was 0.17 cm2, 0.18 cm2, and 0.18 cm2 for the healed, not healed, and entire cohort groups, respectively. There were no significant differences in absolute healing rates. Weekly mean ulcer size for ulcers for the entire cohort, and those who healed and did not heal within 12 weeks are presented in Figure 7.
| Healed by Week-12 | Not-Healed by Week-12 | |
|---|---|---|
| Week-4 decreased by 50% | 5 | 5 |
| Week-4 not decreased by 50% | 1 | 4 |
Table 3: Ulcer size reduction by 50% at Week 4 and healing status at Week 12.
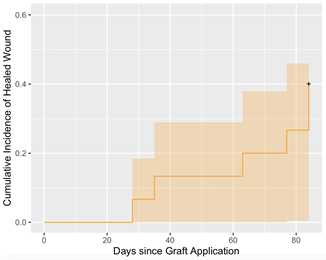
A Kaplan Meier analysis is presented in Figure 8 showing the proportion of healed ulcers at treatment and at each post graft application visit interval with orange shading showing 95% confidence intervals. This analysis reveals a 7% healed rate at week 4 and 40% healed rate at week 12.
Discussion
SOC has been shown to result in an average of 31% DFUs healing in a meta-analysis of controlled trials in weeks [8]. The initial 4 weeks of DFU care have significant predictive value, in that if the percentage area reduction (PAR) is greater than 50%, the wound has a 60% chance of healing in 12 weeks [10]. Conversely, if the PAR is less than 50% in 4 weeks, the wound has a 10% chance of healing in 12 weeks. It is imperative for DFUs to heal before infection develops, which may lead to lower extremity amputation [30]. A number of advanced treatment modalities have been shown to augment and accelerate healing rates in DFUs. Weiman et al. showed that topically applied recombinant human platelet derived growth factor (rh-PDGF-BB) significantly increased the incidence of complete wound closure by 43% and decrease the time to achieve complete wound closure by 32% as compared with placebo controlled gel [31]. Veves et al. demonstrated 56% complete DFU closure at 12 weeks with Apligraf as compared with 38% in the control group [32]. Marston et al. studied the effects of Dermagraft on DFUs and reported 30% complete wound closure by week 12 as compared with 18.3% of control patients [33]. Driver et al. studied the effects of Integra Dermal Regeneration Template (IDRT) on DFUs and found that complete DFU closure during the treatment phase was significantly greater with IDRT (51%) than control (32%) [15]. Additionally, the rate of wound size reduction was 7.2% per week for IDRT subjects vs. 4.8% per week for control subjects. The benefits of using human placental tissues in covering non-healing wounds are now well documented [34,35]. Optimally preserved placental membranes are of particular interest as they contain a combination of growth factors and extracellular matrices as well as viable mesenchymal stem cells (MSCs), fibroblasts, and epithelial cells [36-39]. There are limited, prospective studies on closure and healing rates of cryopreserved umbilical cord graft for slow to heal diabetic foot ulcers [40].
In an open label multi-center trial, Marston et al. studied cryopreserved human umbilical cord in the treatment of complex diabetic foot ulcers complicated by osteomyelitis and demonstrated 50% closure (16/32 patients) in a 16 week study period [41]. In a single center retrospective study, 12 out of 21 wounds (57.1%) achieved closure at 12 weeks [42]. In our study, 40% (6/15) of ulcers had complete closure at 12 weeks. Of the patients whose ulcers did not close by week 12 (9/15 patients, 60%), eight patients had a 50% or greater improvement or by week 12 (88.8%), and five of those ulcers (55.5%) had a closure rate 90% or greater by week 12. Among the entire cohort, fourteen patients (93.3%) had 50% or greater wound reduction by Week 12, and eleven patients (73.3%) patients had 90% or greater wound reduction by Week 12. No significant adverse events were determined to be related to non-DMSO UCG.
The study was limited by enrollment, as there was a drop off rate of 40%, which is slightly high for this type of study. Additionally, this was an open label, non-blinded multi-center study with no active control group, and we used historical data as a comparator. In the future, a larger study enrollment with a control group can be employed to further study this problematic population. Additionally, a head to head comparison of DMSO vs. Non-DMSO UCGs would be helpful to validate superiority and effectiveness.
Conclusion
In the cryopreservation of cells, dimethyl sulfoxide has been widely used as a cryoprotectant. However, it has been shown that DMSO has toxic side effects to the human body, and non-DMSO cryopreservation has been investigated more intensely. The pilot study being presented here studied the effects of non-dimethyl sulfoxide (non-DMSO) viable umbilical cord graft on DFUs. 25 patients were screened for the study, with 15 patients completing the trial. 6/15 (40%) had complete closure prior to or at the study endpoint. This compares well with other cellular/tissue based products available. Among the entire cohort, fourteen patients (93.3%) had 50% or greater wound reduction by Week 12, and eleven patients (73.3%) patients had 90% or greater wound reduction by Week 12. No significant adverse events were determined to be related to non-DMSO UCG. Further studies need to be designed to compare DMSO vs. Non- DMSO UCG effectiveness in slow to heal DFUs. However, based on our findings, non-DMSO viable umbilical cord graft is an effective way to treat challenging DFUs.
Conflict of Interest
None
References
- NCD Risk Factor Collaboration (NCD-RisC). (2016) Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387: 1513–30.
- Centers for Disease Control and Prevention (2014) National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services.
- Harrington C, Zagari MJ, Corea J (2000) A cost analysis of diabetic lower-extremity ulcers. Diabetes Care 23: 1333–1338.
- Petersen M (2013) Economic costs of diabetes in the U.S. in 2012. Diabetes Care 36:1033-1046.
- Driver VR, Fabbi M, Lavery LA (2010) The costs of diabetic foot: The economic case for the limb salvage team. J Vasc Surg 52:17S–22S.
- Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot ulcers in patients with diabetes. J American Med Asso 293:217-228.
- Doupis J, Veves A. (2008) Classification, diagnosis, and treatment of diabetic foot ulcers. Wounds 20:117–26.
- Margolis DJ, Kantor J, Berlin JA (1999) Healing of diabetic neuropathic foot ulcers receiving standard treatment. Diabetes Care 22:692–5.
- Alexiadou K, Doupis J (2012) Management of diabetic foot ulcers. Diabetes Therapy 3(1):4.
- Sheehan P, Jones P, Caselli A (2003) Percent change in wound area of diabetic foot ulcers over a 4 week period is a robust predictor of complete healing in a 12 week prospective trial. Diabetes Care 26(6):1879–1882.
- Papanas N, Maltezos E (2010) Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Safety 33:455-461.
- Kalani M, Jorneskog G, Naderi N (2002) Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers. Long-term follow-up. J Diabetes Complications 16(2):152-258.
- Marston WA, Hanft J, Norwood P, Pollak R (2003) The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 26:1701–1705.
- Brem H, Young J, Tomic-Canic M (2003) Clinical efficacy and mechanism of bilayered living human skin equivalent (HSE) in treatment of diabetic foot ulcers. Surg Technol Int 11:23-31.
- Driver VR, Lavery LA, Reyzelman AM, Dutra TG, Dove CR, et al. (2015) A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound repair and regeneration 23: 891-900.
- Brigido SA (2006) The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: A prospective 16-week pilot study. Int Wound J 3: 181–7.
- Harding K, Kirsner R, Lee D, Mulder G, Serena T (2010) An expert working group review. International consensus: Acellular matrices for the treatment of wounds. London: Wounds International.
- Haugh AM, Witt JG, Hauch A, Darden M, Parker G, et al. (2017) Amnion membrane in diabetic foot wounds: A Meta-analysis. Plast Reconstr Surg Glob Open 5(4):e1302.
- Weng L, Beauchesne PR (2020) Dimethyl sulfoxide-free cryopreservation for cell therapy: A review. Cryobiology 94:9–17.
- Verheijen M, Lienhard M, Schrooders Y, et al. (2019) DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep 9: 4641.
- Shivakumar SB, Bharti D, Subbarao RB, Jang SJ, Park JS, et al. (2016) DMSO†and Serumâ€Free Cryopreservation of Wharton's Jelly Tissue Isolated From Human Umbilical Cord. J Cell Biochem 117: 2397-2412.
- Wang HY, Lun ZR, Lu SS (2011) Cryopreservation of umbilical cord blood-derived mesenchymal stem cells without dimethyl sulfoxide. Cryo Letters 32(1):81-8.
- Ruiz-Delgado GJ, Mancias-Guerra C, Tamez-Gomez EL, Rodriguez-Romo LN, Lopez-Otero A, et al. (2009) Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: Report of three cases and review of the literature. Acta Haematol 122:1–5.
- Morris C, de Wreede L, Scholten M, Brand R, van Biezen A, et al. (2014) Chronic Malignancies and Lymphoma Working Parties of EBMT. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion 54(10):2514-22.
- Windrum P, Morris T CM, Drake MB, Niederwieser D, Ruutu T (2005) Variation in dimethyl sulfoxide use in stem cell transplantation: A survey of EBMT centres. Bone Marrow Transplant 36:601–603.
- Rowlatt U (1979) Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 381(3): 353–361.
- Coolen NA (2010) Comparison between human fetal and adult skin. Archives of Dermatological Research 302(1): 47–55.
- Niknejad H, Peirovi H, Jorjani M (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 15:88-89.
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Moxey PW, Gogalniceanu P, Hinchliffe RJ (2011) Lower extremity amputations: A review of global variability in incidence. Diabet Med 28:1144–53.
- Wieman TT (1998) Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes care 21:822-827.
- Veves A, Falanga V, Armstrong DG, Sabolinski ML (2001) Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes care 24: 290-295.
- Marston WA. Hanft J, Norwood P, Pollak R (2003) The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes care 26:1701-1705.
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA (2012) Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 1: 142-149.
- Brantley JN, Verla TD (2015) Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care (New Rochelle) 4(9):545-559.
- Singer NG, Caplan AI (2011) Mesenchymal stem cells: Mechanisms of inflammation. Ann Rev Pathol 6: 457-478.
- Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, et al. (2010) Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182: 1047-1057.
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A (2009) Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 8: 110-123.
- Duan-Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, et al. (2015) Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle) 4: 523-533.
- Gottrup F, Karlsmark T (2005) Leg ulcers: Uncommon presentations. Clin Dermatol 23 (6): 601-11.
- Marston WA, Lantis JC, Wu SC, Nouvong A, Lee TD, et al. (2019) An open-label trial of cryopreserved human umbilical cord in the treatment of complex diabetic foot ulcers complicated by osteomyelitis. Wound Repair Regen 27(6):680-686.
- Raphael A, Grimes L (2020) Implantation of cryopreserved umbilical cord allograft in hard to heal foot wounds: A retrospective study. J Wound Care 29(Sup8):S12-S17.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2923
- [From(publication date): 0-2021 - Dec 02, 2025]
- Breakdown by view type
- HTML page views: 2202
- PDF downloads: 721