Mini Review Open Access
Aβ Metabolism and the Role of APOE in Alzheimer's Disease
Yue Miao Yin1,3#, Jing Du2# and Zhao Wang1*1MOE Key Laboratory of Protein Sciences, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, People’s Republic of China
2Institute of Biomechanics and Medical Engineering, Department of Engineering Mechanics, School of Aerospace, Tsinghua University, Beijing 100084, People’s Republic of China
3Department of Pharmaceutics, Medical College of Qinghai University, Xining 810001, Qinghai Province, People’s Republic of China
- *Corresponding Author:
- Zhao Wang
MOE Key Laboratory of Protein Sciences
School of Pharmaceutical Sciences
Tsinghua University, Beijing 100084
People’s Republic of China
Tel: +86 10 62772241
E-mail: zwang@tsinghua.edu.cn
Received date: November 02, 2016; Accepted date: November 14, 2016; Published date: November 21, 2016
Citation: Yin YM, Du J, Wang Z (2016) Aβ Metabolism and the Role of ApoE in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 6:285. doi:10.4172/2161-0460.1000285
Copyright: © 2016 Yin YM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Disturbance of the production and clearance of Aβ in the brain is the main cause of memory and cognition decline and contributes strongly to the development of AD. In human, APOE gene has three isoforms, ε2, ε3 and ε4, with APOE ε4 as the most risk gene among them. In the development of AD pathophysiology, ApoE4 is positively associated with Aβ plague formation, but the mechanisms are not clear. In this review, we proposed a hypothesis that the effect of ApoE4 on Aβ possibly involves three processes: 1) ApoE4 can directly interact with Aβ and interferes Aβ clearance. 2) ApoE4 can compete with Aβ for the same receptor, that hinds the cellular uptake pathways of Aβ. 3) ApoE4 also modulates other Aβ degrading proteases like IDE to downregulate Aβ degradation, but the mechanisms needs to be further investigated. These findings suggest that the effect of ApoE in AD pathogenesis is complicated and modulation of ApoE is an attractive strategy for AD therapy.
Keywords
Amyloid-β peptides (Aβ); Apolipoprotein E (ApoE); Alzheimer’s disease (AD)
Introduction
Alzheimer’s disease (AD) is a common neuro degenerative disease associated with cognitive decline and cannot be cured. AD presently affects approximately 13% of people over the age of 65 and 45% over the age of 85 [1], with least 30 million AD patients around the world [2]. Due to an increasing elder population, AD becomes one of the greatest health issues of this century [3]. In 2016, the total health care payments for people age ≥ 65 years with dementia, including long-term care and hospice services, are estimated to be $236 billion [4].
It is widely accepted that neurofibrillary tangles and senile plaques are two hallmarks of AD pathology [5]. Neurofibrillary tangles is associated with Tau hyper phosphorylation while senile plaque involves depositions of aggregated amyloid-β peptides (Aβ) in the gray matter of the brain, mainly in the hippocampus and neocortex [6]. However, the mechanisms of AD occurrence have not been fully elucidated, due to the complex genetic, epigenetic, and environmental factors that may influence of the development of AD. There are two types of AD: Early-onset AD (EOAD) is often familial, with autosomal dominant inheritance, while the vast majority is late-onset Alzheimer’s disease (LOAD) [7]. It is indisputable that the strongest genetic risk factor for LOAD known so far is the human apolipoprotein E (ApoE) gene. Among the three isoforms: ApoE ε2, ApoE ε3, and ApoE ε4, ApoE ε4 increases AD risk about ~3- and 15-fold with a single and double allele respectively [8-10].
Role of Aβ in AD
Normal physiological levels of Aβ is essential to learning and memory as demonstrated by the studies of Morley’s group [11], and low concentration of Aβ has presynaptic enhance effect [12]. Genetic, pathological, and functional researches have provided abundance of evidences that disturbance of the production and clearance of Aβ in the brain is the main cause of Aβ accumulation, aggregation and plague formation, therefor leading to the decline of memory and cognition during the development of AD [13]. Thus, it is not Aβ itself, but the aberrant accumulation of Aβ that is harmful to cognition function.
Amyloid Cascade Hypothesis
Amyloid cascade hypothesis (ACH) has been proposed for almost 25 years. The hypothesis suggests that the deposition of Aβ, which is the major component of the amyloid plaques in AD patients’ brains, is the upstream mediator of AD pathology. Aβ deposition finally leads to neurofibrillary tangles, neuronal loss, cell death, and dementia [14,15]. Currently, a new modified ACH has been proposed by Karran E [16]. The modified ACH considers other hypotheses, such as mitochondrial cascade hypothesis (MCH), vascular hypothesis and Aβ oligomer hypothesis, suggesting that the aggregation of Aβ and tau dysfunction may run in parallel, but the key event in AD pathology is still Aβ deposition.
Aβ Production
Aβ is composed of either 40 or 42 amino acids (Aβ1~40 or Aβ1~42) [17] that generated by amyloid precursor protein (APP). APP is an integral membrane protein of 695-770 AA that is sequentially cleaved by either β-secretase or α-secretase to C-terminal fragment β (CTFβ, 99 AA) or C-terminal fragment (CTFα, 83 AA), then γ-secretase, an intramembrane protease, cleaves CTFβ to Aβ (4 kDa) and CTFα to a fragment named P3 (3 kDa) [18,19]. However, the former pathway brings about the Aβ production is only a small part of APP and the majority (>90%) is the α-secretase pathway [20].
Aβ Degradation and Clearance
Several reviews have elaborated the main mechanisms of Aβ degradation and clearance [21-23]. Two categories of protease are involved in this process: Aβ degrading proteases, which are enzymes that degrade or cleave Aβ into smaller fragments; Extracellular chaperones, which facilitates the transportation of Aβ across the blood brain barrier (BBB) into the blood circulation [24] or astrocyte/ microglia cells [25].
Aβ Degrading Proteasess
Metalloendopeptidases, angiotensin-converting enzyme (ACE), matrix metalloproteinases (MMPs) and lysosomal peptidases are all Aβ degrading protease [26]. Metalloendopeptidases, including neprilysin (NEP), insulin degrading enzyme (IDE) and endothelinconvertingenzymes- 1and-2 (ECE1 and ECE2), play important roles in the degradation of monomeric Aβ species. Deletion of NEP or treatment with an NEP inhibitor leads to increased levels of Aβ [27]. IDE appears to participate in both insulin and Aβ degradation and is mainly expressed in hypothalamic neurons, hippocampus, cerebellum, and brain stem in human [28], and is coinciding with the location of insulin receptors in the brain. Overexpression of NEP and/or IDE declines Aβ level by around 90% and relieves amyloid pathology [29].
Aβ Clearance by Extracellular Chaperones
Extracellular chaperones are proteinsÔľ?which can bind with Aβ in plasma and cerebrospinal fluid (CSF), and are essential because to regulate the formation of Aβ fibrils [30]. These proteins include albumin, α1-antichymotrypsin (ACT), serum amyloid P component (SAP), complement proteins, apoferritin, transthyretin, lipoproteins, and apolipoproteins which includes ApoE.
Role of ApoE in Aβ Metabolism
ApoE is a glycoprotein of 299 AA (34 kDa) that was originally identified as one of the main apolipoproteins which transport lipid from one tissue or cell type to another to regulate lipid homeostasis [31]. In human, ApoE gene exists as three polymorphic alleles-ε2, ε3 and ε4, with the ApoE ε3 allele being the most common (77.9%), ε2 allele the least common (8.4%), and ε4 in the medium (13.7%) [32]. ApoE is an important cholesterol metabolism regulator in the brain. It serves as a cholesterol carrier and mediates the uptake of lipoprotein particles [33]. ApoE is produced by astrocyte or glia cells in brain [34], while it is primarily produced by the liver and macrophages in peripheral tissues, both in humans and animals. ApoE mediates cholesterol metabolism in an isoform-dependent manner [5] because the isoforms have different abilities in binding to ApoE receptors and lipoproteins. It was demonstrated that ApoE4 has greater affinity to very low density lipoproteins (VLDL), while ApoE3 and ApoE2 have a preference for small high-density lipoproteins (HDLs) [35,36]. In addition, ApoE isoforms also affect synaptic plasticity in an isoformdependent manner. ApoE3 promotes neurite outgrowth and increase neuronal sprouting [37]. However the studies of the effect of ApoE4 on synaptic plasticity was controversial. Teter’s study reported that ApoE4 had prejudicial effects on neurite outgrowth [38], while Puttfarcken’ study suggested ApoE4 even had stimulating effects in the absence of Aβ [39].
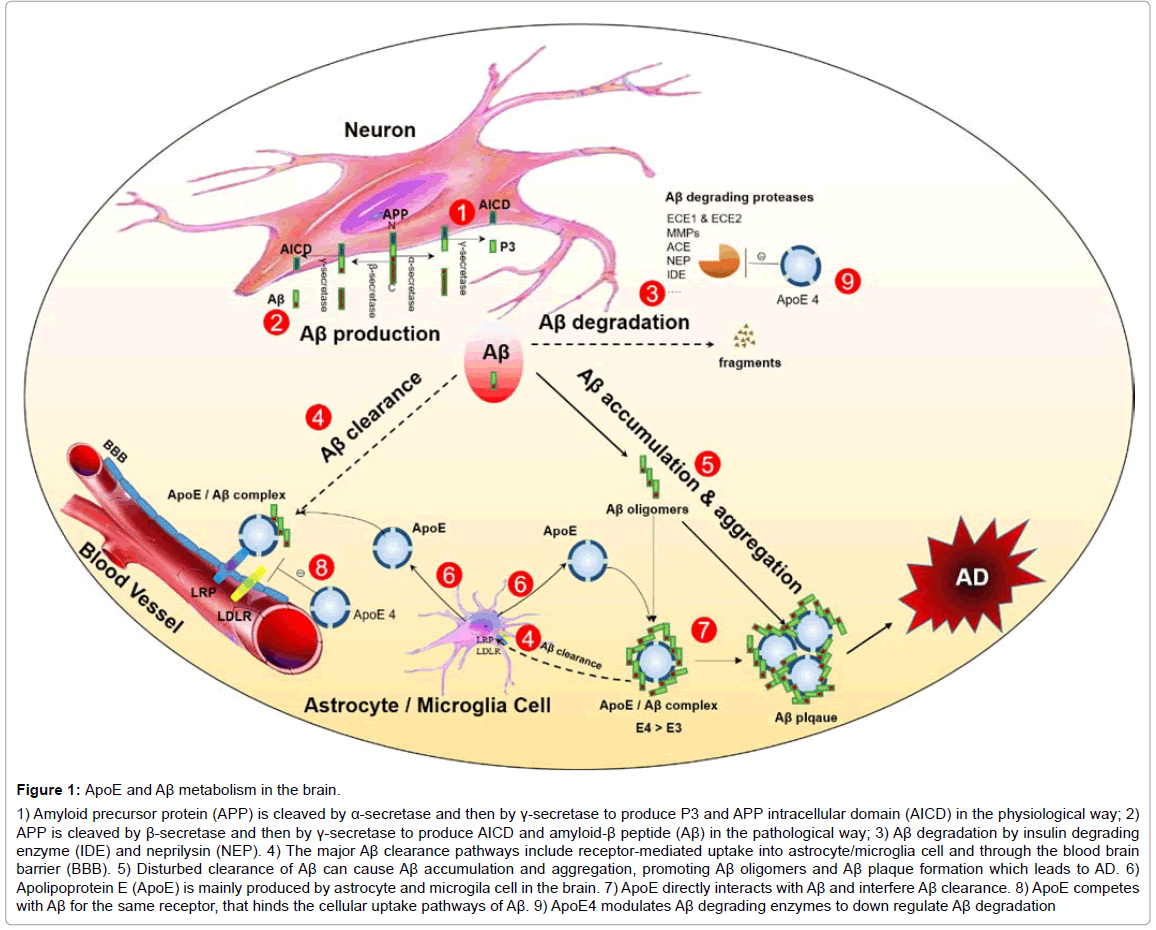
Figure 1: ApoE and Aβ metabolism in the brain.
1) Amyloid precursor protein (APP) is cleaved by α-secretase and then by γ-secretase to produce P3 and APP intracellular domain (AICD) in the physiological way; 2) APP is cleaved by β-secretase and then by γ-secretase to produce AICD and amyloid-β peptide (Aβ) in the pathological way; 3) Aβ degradation by insulin degrading enzyme (IDE) and neprilysin (NEP). 4) The major Aβ clearance pathways include receptor-mediated uptake into astrocyte/microglia cell and through the blood brain barrier (BBB). 5) Disturbed clearance of Aβ can cause Aβ accumulation and aggregation, promoting Aβ oligomers and Aβ plaque formation which leads to AD. 6) Apolipoprotein E (ApoE) is mainly produced by astrocyte and microgila cell in the brain. 7) ApoE directly interacts with Aβ and interfere Aβ clearance. 8) ApoE competes with Aβ for the same receptor, that hinds the cellular uptake pathways of Aβ. 9) ApoE4 modulates Aβ degrading enzymes to down regulate Aβ degradation
Furthermore, ApoE also related to Aβ metabolism in AD in isoform-dependent manner. In AD patients, compared to ε2 and ε3, the presence of ε4 is associated with increased risk for both EOAD and LOAD, especially LOAD. Studies have demonstrated that there is a strong link between ApoE ε4 and the pathology of neural disorders in AD [40]. Genetic studies have found that the risk of suffering from AD by 85 years of age among persons who inherit double ε4 alleles is 50- 90%, and the probability among persons with one ε4 allele is 45% [41].
Although the linkage between ApoE ε4 gene and the increased risk of AD is obvious, the mechanism for effect of ApoE in AD is complex, since ApoE is associated with many aspects of AD, including Aβ plaque formation, inflammation, oxidative stress, synaptic plasticity loss, cholinergic dysfunction, and lipid homeostasis deregulation [42]. There are evidences to indicate that levels of soluble Aβ are increased with ApoE4, providing a potential mechanism of ApoE4-induced AD risk [43]. However, the pathway(s) by which ApoE4 may increase Aβ levels are unclear.
Role of ApoE in Aβ Accumulation
It is clear that ApoE can directly interact with Aβ. Histological analyses of AD patients’ brains show that ApoE is co-deposited with Aβ in amyloid plaques [44]. Epitope mapping demonstrates that residues 13-17 in Aβ and residues 144-148 in the ApoE N-terminal region are interacting with each other, forming the ApoE/Aβ complexes [5]. Purified ApoE4 binds Aβ with a higher affinity than ApoE3, but this affinity is reversed when using lipidated ApoE [45,46]. Researches have shown that ApoE increases the level of Aβ oligomers in an isoformdependent manner (ApoE4 > ApoE3 >ApoE2) [47,48]. Moreover, blocking the ApoE/Aβ interaction, Aβ-related pathology is mitigated: reduced brain Aβ- accumulation, co-accumulation of ApoE within Aβ plaques and neuritic degeneration in both APP/E2 and APP/E4 mice [49].
Role of ApoE in Aβ Clearance and Degradation
It has been proposed that ApoE can indirectly modulate Aβ clearance. All three isoforms of ApoE present obstructing effect on Aβ cellular uptake pathway, by competing with Aβ for the same receptors such as low density lipoproteins (LDL) receptor-related protein (LRP) in astrocytes [50].
Our previous report has shown that ApoE also regulates Aβ degradation by IDE extracellularly. ApoE4 significantly reduces the expression of IDE, while ApoE3 could rescue this down-regulation in ApoE knockout (apoe−/− mice). These effects on IDE expression by ApoE can be prevented by receptor - associated protein (RAP), which blocked the interaction between ApoE and members of the LDL receptor family [51], suggesting that various ApoE isoforms could exert different effect on IDE via membrane receptor. Keeney’ research demonstrated that ApoE4 mice exhibited downregulated IDE and peroxisome proliferator-activated receptor (PPARγ) levels [52]. In another paper from our lab, we showed that PPARγ could transcriptionally activate IDE gene expression [53]. These results indicate that ApoE4 may reduce IDE expression by inhibiting PPARγ. Meanwhile, when compared to ApoE2 mice brain, both ApoE3 and ApoE4 mice brain showed significantly decreased insulin /insulin-like growth factor 1 (Igf1), insulin receptor substrates (Irs) and facilitated glucose transporter 4 (Glut4) expression, suggesting that ApoE isoforms differentially modulate the expression of major players involved in Igf1 signaling and glucose and Aβ metabolism [52]. Other research proved that ApoE4 was significantly less efficient in promoting the degradation of soluble Aβ compared to ApoE2. In addition, lipidated ApoE showed stronger effects on degrading Aβ than non-lipidated ApoE by affecting the capacities of IDE [54].
Acknowledgment
This work was Ô¨Ānancially supported by the National Basic Research Program of China (973 Program, 2013CB530802) and the National Natural Science Foundation of China (No.81270425, No. 81471396).
References
- Alzheimer’s Association (2012) 2012 Alzheimer’s disease facts and figures. Alzheimer’s dement 8: 131-168.
- Hung AS, Liang Y, Chow TC, Tang HC, Sharon LY, et al. (2016) Mutated tau, amyloid and neuro inflammation in Alzheimer disease—A brief review. Prog Histochem Cytochem 51: 1-8.
- Hickman RA, Faustin A, Wisniewski T (2016) Alzheimer disease and its growing epidemic risk factors, biomarkers and the urgent need for therapeutics. Neurologic Clinics 34: 941-953.
- Alzheimer's Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimer’s dement 12: 459-509.
- Kanekiyo T, Xu H, Bu G (2014) ApoE and Aβ in Alzheimer's disease: Accidental encounters or partners? Neuron 81: 740-754.
- Jinghui L, Sebastian W, Astrid G, Abrahams JP (2016) Cross-interactions between the Alzheimer disease amyloid-β peptide and other amyloid proteins: A further aspect of the amyloid cascade hypothesis. J Biol Chem 291: 16485-16493.
- Šimiń? G, Babiń? Leko M, Wray S, Harrington CR, Delalle I, et al. (2016) Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol S0301-0082: 30089-30097.
- Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, et al. (2012) Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135: 2155-2168.
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, et al. (1993) Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43: 1467-1472.
- Kim D, Tsai LH (2009) Bridging physiology and pathology in AD. Cell 137: 997-1000.
- Morley JE, Farr SA (2014) The role of amyloid-β in the regulation of memory. Biochem Pharmacol 88: 479-485
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, et al. (2009) Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12: 1567-1576.
- Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med 2: a006338.
- Hardy JA, Higgins GA (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256: 184-185.
- Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6: 487-498.
- Karran E, De Strooper J (2016) The amyloid cascade hypothesis: Are we poised for success or failure? Neurochem139: 237-252.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Bright J, Hussain S, Dang V, Wright S, Cooper B, et al. (2015) Human secreted tau increases amyloid-beta production. Neurobiol Aging 36: 693-709.
- Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, et al. (2015) Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease. Curr Alzheimer Res 12: 32-46.
- Sambamurti K, Greig NH, Utsuki T, Barn well EL, Sharma E, et al. (2011) Targets for AD treatment: Conflicting messages from gamma-secretase inhibitors. J Neurochem 117: 359-374.
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, et al. (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11: 457-470.
- Yoon S, Jo SA (2012) Mechanisms of amyloid-β peptide clearance: Potential therapeutic targets for Alzheimer’s disease. Biomol Ther 20: 245-255.
- Saido T, Leissring MA (2012) Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med 2: a006379.
- Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8: 16-30.
- Kanekiyo T, Cirrito JR, Liu CC, Shinohara M, Li J, et al. (2013) Neuronal clearance of amyloid-β by endocytic receptor LRP1. J Neurosci 33: 19276-19283.
- Ries M, Sastre M (2016) Mechanisms of Aβ clearance and degradation by glial cells. Front Aging Neurosci 8: 160.
- Farris W, Schütz SG, Cirrito JR, Shankar GM, Sun X, et al. (2007) Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am J Pathol 171: 241-251.
- Bernstein HG, Lendeckel U, Bukowska A, Ansorge S, Ernst T, et al. (2008) Regional and cellular distribution patterns of insulin-degrading enzyme in the adult human brain and pituitary. Chem Neuroanat 35: 216-224.
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, et al. (2003) Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology and premature death. Neuron 40: 1087-1093.
- Bates KA, Verdile G, Li QX, Ames D, Hudson P, et al. (2009) Clearance mechanisms of Alzheimer’s amyloid-β peptide: Implications for therapeutic design and diagnostic tests. Mol. Psychiatry 14: 469-486.
- Mahley RW, Rall SC Jr (2000) Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1: 507-537.
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, et al. (2007) Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 278: 1349-1356.
- Hirsch-Reinshagen V, Burgess BL, Wellington CL (2009) Why lipids are important for Alzheimer disease? Mol Cell Biochem 326: 121-129.
- Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and Therapy. Nat Rev Neurol 9: 106-118.
- Mahoney-Sanchez L, Belaidi AA, Bush AI, Ayton S (2016) The complex role of apolipoprotein E in Alzheimer's disease: An overview and update. J Mol Neurosci 60: 325-335.
- Huang Y, Mahley RW (2014) Apolipoprotein E: Structure and function in lipid metabolism, neurobiology and Alzheimer's diseases. Neurobiol Dis 72(Pt A): 3-12.
- Kim J, Yoon H, Basak J, Kim J (2014) Apolipoprotein E in synaptic plasticity and Alzheimer's disease: Potential cellular and molecular mechanisms. Mol Cells 37: 767-776.
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, et al. (2002) Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. J Neurosci Res 68: 331-336.
- Puttfarcken PS, Manelli AM, Falduto MT, Getz GS, LaDu MJ (1997) Effect of apolipoprotein E on neurite outgrowth and beta-amyloid-induced toxicity in developing rat primary hippocampal cultures. J Neurochem 68: 760-769.
- Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA 103: 5644-5651.
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, et al. (2006) Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26: 4985-4994.
- Arbor SC, LaFontaine M, Cumbay M (2016) Amyloid-beta Alzheimer targets - protein processing, lipid rafts and amyloid-beta pores.Yale J Biol Med 89: 5-21
- Tai LM, Mehra S, Shete V, Estus S, Rebeck GW, et al. (2014) Soluble apoE/Aβ complex: Mechanism and therapeutic target for APOE4-induced AD risk. Mol Neurodegener 9: 2.
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 541: 163-166.
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, et al. (1994) Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem 269: 23403-23406.
- LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, et al. (1995) Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem 270: 9039-9042.
- Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, et al. (2012) Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid b peptide. J Neurosci 32: 15181-15192.
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, et al. (2012) APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 287: 41774-41786.
- Pankiewicz JE, Guridi M, Kim J, Asuni AA, Sanchez S, et al. (2014) Blocking the apoE/Aβ interaction ameliorates Aβ-related pathology in APOE ε2 and ε4 targeted replacement Alzheimer model mice. Acta Neuropathol Commun 2: 75.
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, et al. (2013) ApoE influences amyloid-beta (A beta) clearance despite minimal apoE/A beta association in physiological conditions. Proc Natl Acad Sci USA 110: E1807-E1816.
- Du J, Chang J, Guo S, Zhang Q, Wang Z (2009) ApoE 4 reduces the expression of A beta degrading enzyme IDE by activating the NMDA receptor in hippocampal neurons. Neurosci Lett 464: 140-145.
- Keeney JT, Ibrahimi S, Zhao L (2015) Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: Evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer's disease prevention and early intervention. J Alzheimers Dis 48: 411-424.
- Du J, Zhang L, Liu S, Zhang C, Huang X, et al. (2009) PPARγ transcriptionally regulates the expression of insulin-degrading enzyme in primary neurons. Biochem Biophys Res Commun 383: 485-490.
- Jiang Q, Lee CY, Mandrekar S (2008) ApoE promotes the proteolytic degradation of A beta. Neuron 58: 681-693.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4933
- [From(publication date):
November-2016 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 4109
- PDF downloads : 824