A Meta-Analysis on the Association of Vitamin B, D, and E Levels with the Progression and Risk of Alzheimer's Disease
Received: 19-Oct-2022 / Manuscript No. JADP-22-77789 / Editor assigned: 21-Oct-2022 / PreQC No. JADP-22-77789 (PQ) / Reviewed: 04-Nov-2022 / QC No. JADP-22-77789 / Revised: 11-Nov-2022 / Manuscript No. JADP-22-77789 (R) / Published Date: 18-Nov-2022 DOI: 10.4172/2161-0460.1000555 QI No. / JADP-22-77789
Abstract
Dementia describes a group of clinical symptoms including memory loss and personality changes as the disease affects the processing of information by the brain. The most common cause of dementia is Alzheimer’s Disease (AD). Despite decades of research, there are currently no curative interventions for AD and no preventive strategies. Vitamins are essential nutrients required for normal bodily function, and so accordingly lack of adequate amounts of vitamins (vitamin deficiency) is associated with many health issues. This proposal builds on the statistical analysis of the results of multiple studies combined (a meta-analysis) to investigate the association of AD with vitamins B, D, and E. I found that deficiency of each vitamin may be related to increased risk and/or worsened progression of AD. This correlation relates to these vitamins being directly and indirectly involved in processes that prevent damage and destruction of the brain, an important part in AD initiation and progression.
This research project will determine if the pharmacological supplementation of vitamins B, D, and E will alleviate the severity and halt the progression of AD, which would be a widely beneficial and cost–effective approach in tackling a major health issue.
Keywords: Dementia; Alzheimer’s disease; Vitamin deficiency; Hyperhomocysteinaemia
Introduction
Alzheimer's Disease (AD) is a progressive neurodegenerative disease and the most prominent cause of dementia in elderly people. AD is classed as a protein misfolding disease, majorly associated with plaque accumulation of abnormally folded and insoluble amyloid β protein outside and around neurons, alongside neurofibrillary tangles of tau within cells. This causes loss of neuronal connections in the brain seen as memory loss, disorientation, and an overall decline in cognition.
The recent failure of clinical trials based around anti-amyloid therapies that target either the production (verubecestat) or clearance of (solanezumab) Aβ, are disappointing and demonstrates that there is an urgent need to explore alternative therapeutic avenues that fall outside the traditional amyloid cascade hypothesis. Vitamins could be a potential therapeutic avenue to tackle the many complications of AD by slowing its neurodegenerative progression and potentially reducing its risk of initiation. Vitamins are organic compounds with diverse biochemical functions, with vitamins B, D, and E in particular being suggested by a plethora of studies to have roles in neuroprotection in the context of AD.
Vitamin B reduces blood homocysteine levels, important as hyperhomocysteinaemia (blood levels above 15 μmol/L) is related to atrophy of key brain regions related to cognitive decline in AD; researchers found that each μM increase in blood homocysteine level raised AD risk by 16%, whilst each pM increase in vitamin B12 was associated with a 2% reduction elderly AD risk. Vitamin B9 (folic acid) has roles in cognitive and social function, with deficiency contributing to brain ageing processes; a prospective study of 370 healthy subjects found that this deficiency doubled the risk of developing AD. Vitamin D stimulates the phagocytic clearance of amyloid plaques to reduce cytotoxicity and apoptosis associated with AD progression. As an antioxidant, vitamin E lowers the increased amount of reactive species and oxidative damage in the brain that impairs cognitive function in AD patients. To investigate the potential effect of vitamins against AD pathology and further treatment options, I conducted a meta-analysis on changes in cognitive status (based on MMSE scores) and risk (via hazard ratios) with vitamin status. Using fixed and random effects models, I found that folic acid treatment may not increase average MMSE scores, whilst vitamin D deficiency gives a higher AD hazard ratio, and vitamin E levels were lower in AD patients than healthy control subjects. Overall, results indicated vitamin deficiency increasing AD risk and cognitive decline, with the possibility of improvement by vitamin treatment.
Combined or partial vitamin B, D, and E supplementation in deficient AD patients and at-risk individuals will help reduce the progression of AD and lower risk through various mechanisms as a potential therapeutic option. I will demonstrate how vitamins B, D, and E may protect against AD pathology progression via improvements in cognitive status as measured by MMSE scores, and how these vitamins can lower risk based on the hazard ratio of healthy control subjects with vitamin deficient AD patients.
Scientific case
Alzheimer's Disease (AD) is a progressive neurodegenerative disease and the most prominent cause of dementia in older people, encompassing 60%–70% of all-cause cases. Debilitating symptoms of memory loss, behavioral issues, and loss of bodily functions severely reduce patient quality of life and life expectancy. Despite its current prevalence and extensive morbidity, no available treatments can reverse or effectively prevent its progression– only temporarily improve symptoms, and the cases are expected to only further rise [1]. AD is classed as a protein misfolding disease, specifically associated with plaque accumulation of abnormally folded and insoluble amyloid β protein outside and around neurons, alongside neurofibrillary tangles of tau within cells [2]. Established risk factors include age, polygenic family history, and traumatic brain injury. Lifestyle factors such as diet type and vitamin intake potentially play an important role in AD progression and severity.
Vitamins are organic compounds with diverse biochemical functions, required in small quantities for proper metabolic function in humans. Required amounts come from dietary intake as the body either does not produce them or produces very little, and where dietary deficiency occurs a range of pathologies arise [3]. There is a particular focus on vitamins B, D, and E given extensive evidence and suggested mechanisms linking their functional roles in the body to the prevention and alleviation of AD hallmarks and symptoms. Vitamin B indirectly plays a role by reducing levels of homocysteine, which at high amounts is linked to the mental declines associated with AD [4-6]. Vitamin D more directly acts by stimulating the phagocytic clearance of amyloid plaques to reduce cytotoxicity and apoptosis associated with AD progression [7-9]. Finally, as an antioxidant vitamin E counteracts the increased concentration of reactive species and oxidative damage in the brain commonly seen in AD patients, improving cognitive function after achieving this effect [10,11].
To increase the chance of detecting a statistically significant, real effect of these vitamins in AD patients, I conducted a meta–analysis as a combination of several studies increases the chance of detecting an effect (i.e. the power). Additionally, a meta–analysis improves the precision of estimating the effects of vitamin intervention, overall providing a better investigation into the potential effects of vitamins against AD pathology. In this meta–analysis, I explored and evaluated the relationship between vitamin B, D, and E deficiency and AD severity, progression, and risk.
Given that regular vitamin intake is affordable and holistically beneficial for one’s health, the link between AD progression and severity with vitamin deficiency is of significant importance– with the implication that vitamin sufficiency can provide an affordable, convenient, and universally beneficial solution for the individual and systematic healthcare pressures of AD.
Selection of vitamins
The study describes about the meta–analysis on the aforementioned vitamins based on many factors. Primarily, there are large amounts of robust evidence providing a firm foundation for their roles in AD, and the promise of utilizing these to the fullest extent possible. Additionally, Vitamin B and D are relatively deficient throughout various geographic and ethnic populations, and as these are fairly avoidable deficiencies with potentially severe outcomes such as increased AD risk, further evidence to promote vitamin sufficiency will certainly benefit a range of individuals. Vitamins B and E deficiencies are also linked to AD progression whilst vitamin D deficiency is more closely related to AD risk, and so rather than focusing on one vitamin’s particular effects I decided a more holistic approach could be more fruitful in tackling a research gap. The varied effects on different aspects of AD’s pathology and prognosis are possible due to their varied links to AD. Firstly, circulating concentrations of the amino acid homocysteine above the normal upper limit of 15 μM were histologically confirmed to be associated with AD progression both indirectly and directly. Indirectly, hyperhomocysteinaemia is an independent risk factor for brain infarcts in stroke, which AD commonly co-occurs with [4]; directly, elevated homocysteine disturbs redox potentials and promotes amyloid and tau protein accumulation, apoptosis, and the neuronal death seen in AD [5]. Serum homocysteine levels are normally regulated by conversion to methionine using vitamin B12 and folic acid (vitamin B9) as cofactors, and so accordingly deficiencies in either vitamin may cause hyperhomocysteinaemia and result in an increased AD risk [6]. It was shown that even where vitamin intake is sufficient, homocysteine levels can be reduced by administration of high-dose supplements of folic acid, vitamin B6, and vitamin B12, providing a potential avenue for treatment and prevention [4,6]. Secondly, low serum vitamin D concentrations are associated with prevalent AD, likely due to the in vitro proven role of vitamin D in the brain; through macrophage stimulation, vitamin D increases the phagocytic clearance of amyloid plaques to the effect of reducing cytotoxicity and apoptosis in primary cortical neurons [7]. Furthermore, prospective studies show that low concentrations in the elderly are associated with increased risk of cognitive decline, supported by findings of the receptor for its active form (1,25-dihydroxyvitamin D3) and the enzyme that synthesizes it (1α-hydroxylase) being located throughout the brain [8]. Additionally, vitamin D deficiency has been linked to ischemic stroke risk and brain atrophy, highly linked to AD progression [9].
Lastly, deficiency of the antioxidant vitamin E increases risk of AD (particularly in elderly people) whilst sufficient levels were reported to slow progression and suppress tau-induced neurotoxicity [10]. Given that a hallmark of AD is elevated oxidative damage, with increased concentrations of oxidized nucleic acids, proteins, and lipids found in AD brains, the role of vitamin E in scavenging and neutralizing reactive oxygen/nitrogen species could provide a causal link for its significance in AD development and progression [11].
Materials And Methods
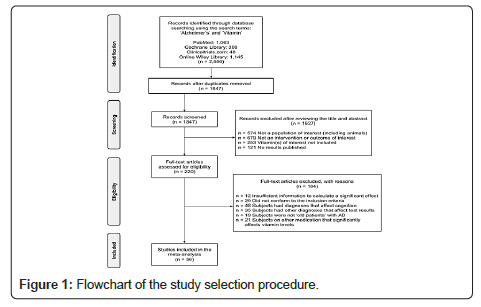
The initial series of electronic searches were conducted between December 2020 and February 2021 and yielded 2556 potentially relevant clinical trials and literature. Search keywords included ‘Alzheimer’s’ and ‘Vitamin’. After duplicates were removed, 1847 citations remained on the basis of their titles and abstracts. Of these, 220 under-went full-text screening. After the screening process, I then conducted eligibility checks, ultimately producing 56 studies that met the inclusion criteria to be included in the meta-analysis, including 22 clinical studies with data on patient serum values, mental state, and mental examination scores (Figure 1).
The initial stage of research will systematically investigate vitamin levels in AD patients and aim to identify any association with MMSE scores, providing results that a series of randomized, double- blind, placebo–controlled trials can be based on. In the vitamin B arm, 100 male and female patients aged >55years with mild to moderate AD and will be randomly assigned to concurrently take 1 mg of methylcobalamin and 5 mg of folic acid, or placebo once daily for 24 months. In the vitamin D arm, 100 male and female patients aged >55years with mild to moderate AD will be randomly assigned to concurrently take 1,000mg of calcium carbonate combined with 10micrograms IU of vitamin D (3), or placebo once daily for 24 months. In the vitamin E arm, 100 male and female patients aged >55years with mild to moderate AD will be randomly assigned to concurrently take 15 mg of alpha-tocopherol, or placebo once daily for 24 months.
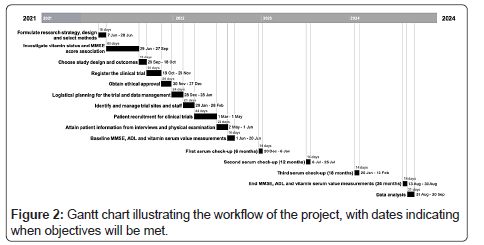
At the beginning of this trial, MMSEs will be performed, and the scores recorded for reference, alongside notes of patient ADL level of difficulty. Serum measurements will also be taken at baseline and every six months during the study. At the end of the 24 months, MMSEs will be taken, and the score differences compared. This will be the primary outcome measure and act as a quantitative measure for changes in cognition. Patients with deficiencies will be noted and additionally have their MMSE separately compared to identify if vitamin supplementation has a greater impact when deficiency is present. Secondary outcomes will also include other components of AD, namely assessing changes in the performance of Activities of Daily Living (ADLs) and concentrations of homocysteine (for vitamin B group only). Analysis of this collected data will then occur to explore the aforementioned objectives. The Figure 2 displays a Gantt chart illustrating the workflow of the project.
Study selection and data extraction
Given the different mechanistic links between the different vitamins in AD pathogenesis and progression, the included studies compared different correlations between vitamin deficiency and AD, but still met the same general inclusion criteria. These criteria included: the study design was based on an at-risk/‘old’ human population, with a mean age of at least 55 years old; the studies described the assessment standard of AD; the studies provided sufficient information to calculate effect size and significant effects; cases and controls had no vitamin B/D/E supplement intake or medication that would significantly affect vitamin serum/plasma concentrations; cases and controls were not diagnosed with other conditions that significantly affect cognition and results (as potential confounding factors).
Additionally, data on the effect of folic acid came from randomized, case-control trials in elderly patients with poor cognitive faculty, secondary to AD, and who received homocysteine-lowering B vitamins or supplements containing vitamin B6, B12, and folic acid as adjunctive therapy. I excluded data on single-arm studies and trials in healthy participants.
The Vitamin D data selection came from community–based prospective cohort studies, with subjects free of dementia at baseline; additionally, criteria included measurement of serum vitamin D as exposure variable and risk measure information with the 95% CI for the risk of all-cause dementia. Selected model data was adjusted for at least: age, sex, education, income, body mass index, risk factors including smoking status, diabetes, history of cardiovascular disease, and homocysteine. Finally, the Vitamin E data selection used case‐control studies that ensured all cases and controls had no additional vitamin E intake, alongside the mentioned exclusion criteria.
Quality assessment
Reporting results from various resources holds the potential for erroneous conclusions to be presented by the author or drawn by the reader where quality is not properly assessed. This is especially important in a meta–analysis such as this, where incorrect conclusions may be used to guide clinical decisions with the potential for ineffectual or harmful treatment. To minimise the potential for this problematic misinterpretation of data, I used the Newcastle Ottawa Scale (NOS) [12] to assess the quality of the studies selected for primary analysis based on three broad features: study objective selection, study group comparability, and exposure or outcome.
For the cohort studies in vitamin D analysis, a study was awarded a maximum of one star for each numbered item within the selection and outcome categories, and a maximum of two stars for comparability. The case-control studies in the vitamin B and E analyses were awarded a maximum of one star for each numbered item within the selection and exposure categories, and a maximum of two stars can be given for comparability. In each case, individual studies could have a maximum quality rating of nine stars, and out of this total I consider a score of ≥ 7 as high quality, 5-6 as moderate quality, and ≤ 4 as low quality. NOS scores are in the following ‘study characteristics’ section.
Statistical analysis
I used Google Colaboratory software to manage data and calculate the pooled estimation, specifically using coding languages R version 4.0.0 [13,14] and Python version 3.7 [15] to achieve x statistical computing and graphics. Given considerable heterogeneity, I completed the analyses with a random effects model. This model assumes the studies were drawn from populations that differ in ways that could impact the treatment effect and effect size (i.e. heterogenous), including random error within studies and true variation in effect size between the studies [16]. When no significant heterogeneity was detected, I performed a fixed‐effects model. This assumes that all studies in the meta-analysis share a common true effect size where all factors which could influence the effect size are the same in all the study populations. This assumes that variation observed in studies’ effect sizes are only due to the random inherent error in each study [16]. Forest plots were used to depict the results graphically [17]. For the initial investigation based on MMSE data, heterogeneity, pooled estimation, and summary effect statistical analyses are required. Changes in mean MMSE scores can be calculated and analyzed using Google Colaboratory software with coding languages R version 4.0.0 [13,14] and Python version 3.7 [15]. Publication bias must also be identified, possible through visual inspection of funnel plots produced by Egger’s test in the statistical software RevMan 5.4.1. Additionally, sensitivity analysis to assess the influence of each study on the overall pooled effect size should be performed. For the clinical trials, these same methods and resources can be used to analyses MMSE data.
Publication bias and sensitivity analyses
Publication bias is the tendency of authors to publish studies with significant results based on the experiment and research study outcomes [18]. This bias can be identified by visually inspecting the funnel plots produced by Egger’s test in the statistical software RevMan 5.4.1[19]. This performs a linear regression of intervention effects estimated on the standard error weighted by inverse variance. The product is a funnel plot, with symmetricity indicating no publication bias, as the effect size and sample size are not dependent [18]. This was important to perform because publication bias means that certain published studies are not true representatives of available evidence, with the potential to distort the results of the meta-analysis. Wider implications are false impressions wasting research opportunities, time, and money [20].
To assess the influence of each study on the overall pooled effect size, sensitivity analysis was performed by repeating the random effects model after omitting one study at a time to assess whether the summary risk effect was biased by the effect of any individual study. A p ≤ 0.05 was considered statistically significant. This was important to do in order to explore the impact of different decisions on results, and whether results were robust (being the same/similar with different decisions) or need to be interpreted with caution. This can be performed in the same software [19].
Results
Study characteristics
Out of a total of 2556 studies that contained search words, 56 studies were included for analysis based on the selection criteria (Figure 1). Of these, 22 contained the necessary data to be used in the initial forest plot analysis. Most studies were conducted in developed countries. The number of participants in each dataset ranged from 11 to 10,186 with a mean of 1034; in the prospective cohort studies, the duration of follow-up ranged from 3 months to 21 years; overall, study NOS scores ranged from 5 to 8. The tables below display the study characteristics according to their vitamin of focus (Tables 1-3).
| Author (Year) | Total Sample Size (all AD patients) | Female % | Mean Participant Age ± SD in years at baseline | Study Duration (months) | Active treatment (oral unless specified) | Control Group Change in Mean Score on MMSE ± SD from baseline | Treatment Group Change in Mean Score on MMSE ± SD from baseline | Mean Score Difference | Standard Error (SE) | Lower Limit, Upper Limit | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aisen (2008) [21] | 409 | 56 | 76.3 ± 8.0 | 18 | 5 mg folic acid, 1 mg B12, 25 mg B6 | −3.08 ± 4.46 | −2.65 ± 4.56 | 0.43 | 0.215 | -0.503,1.363 | 6 |
| Connelly (2008) [23] | 41 | 70.7 | 76.27 ± 6.23 | 6 | 1 mg folic acid, cholinesterase inhibitor | 0.22 ± 2.67 | 0.09 ± 3.30 | -0.13 | 0.065 | -2.006,1.746 | 8 |
| Kwok (2011) [51] | 99 | 63.6 | 78 | 23 | 5 mg folic acid, 1 mg B12 | 2.8 ± 5.2 | 2.1 ± 3.7 | -0.7 | 0.35 | -1.374, 1.374 | 6 |
| Sun (2007) [52] | 89 | 49.4 | 75 ± 7.3 | 6.5 | 5 mg B6, 0.5 mg B12, 1 mg folic acid | 0.41 (-1.12;1.93) | 0.15 (-1.06;1.35) | -0.26 | 0.13 | -1.105,0.585 | 7 |
Table 1a: Characteristics of vitamin B studies (focused on folic acid).
| Author (Year) | Total Sample Size (all AD patients) | Female % | Mean Participant Age in years at baseline | Study Duration (months) | Active treatment (oral unless specified) | Placebo Group Change in Mean Score on MMSE ± SEM from baseline | Treatment Group Change in Mean Score on MMSE ± SEM from baseline | NOS Score |
|---|---|---|---|---|---|---|---|---|
| Blass (1988) [53] | 11 | 63.6 | 71.6 | 3 | 3 g thiamine, nicotinamide placebo | 0.54 ± 0.68 | 1.35 ± 0.67 | 5 |
Table 1b: Characteristics of vitamin B studies (focused on nicotinamide).
| Author (Year) | Total Sample Size (all AD patients) | Female % | Mean Participant Age in years at baseline | Study Duration (months) | Active treatment (oral unless specified) | Placebo Group Change in Mean Score on VF | Treatment Group Change in Mean Score on VF | Placebo Group Change in Mean Score on HVLT disc | Treatment Group Change in Mean Score on HVLT disc | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Kay (2014) [57] | 17 | N/A | 77.5 | 6 | 10 mg stabilized oral NADH | -3.63 | 1.53 | -1.75 | 2.57 | 7 |
Table 1c: Characteristics of vitamin B studies (Placebo Group Change in Mean Score on HVLT disc).
| Authour (Year) | Total Sample Size (all AD patients) | Female % | Mean Participant Age in years at baseline | Study Duration (months) | Active treatment (oral unless specified) | ADAS-Cog TS | CDR Sum | ADCS ADL TS (Instrumental) |
MMSE TS | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Phelan (2017) [55] | 31 | 32.3 | 79.4 | 6 | 1500 mg nicotinamide twice daily | -0.25 (-3.29;2.78) | -0.46 (-1.41;0.48) | 0.60 (- 3.96;5.15) | –0.90 (- 2.93;1.12) | 7 |
Table 1d: Characteristics of vitamin B studies (Estimated Treatment-Control Mean Difference (95-CI)).
| Author (Year) | Total Sample Size | Female % | Mean Participant Age ± SD in years at baseline | Mean Follow-up (years) | Mean Serum Vitamin D (ng/mL) ± SD | AD Patient Sample Size | AD Patients with Vitamin D Deficiency | Cutoff for Vitamin D deficiency (ng/mL) | Definition of adequate Vitamin D (ng/mL) | Multivariable adjusted HR | P value | 95%-CI | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karakis (2016) [22] | 1663 | 58.5 | 72.4 ± 6.7 | 9 | 25.1± 11.4 | 208 | <96 | <10 | 20-50 | 0.97 | 0.93 | 0.47;2.00 | 6 |
| Feart (2017) [28] | 916 | 62.3 | 73.3 ± 4.5 | 10.8 | 14.3 ± 6.7 | 124 | 43 | <10 | >20 | 2.85 | 0.017 | 1.37;5.97 | 7 |
| Littlejohns (2014) [29] | 1658 | 69.2 | 73.6 ± 4.5 | 5.6 | 25.8 ± 10.6 | 100 | <70 | <10 | >20 | 2.22 | 0.008 | 1.02;4.83 | 8 |
| Afzal (2014) [57] | 10,186 | 56.1 | 57.6 | 21 | 16.4 | 418 | 93 | <10 | >20 | 1.25 | 0.11 | 0.95;1.64 | 7 |
| Licher (2017) [58] | 6087 | 56.9 | 69.2 | 13.3 | 19.6 | 641 | N/A | <10 | >20 | 1.13 | N/A | 1.03;1.24 | 6 |
Table 2: Characteristics of vitamin D studies.
| Author (Year) | Total Sample Size | Female % | Mean Control Age ± SD in years at baseline | Mean AD Age ± SD in years at baseline | Control Serum Vitamin E (μmol/l) ± SD | AD Serum Vitamin E (μmol/l) ± SD | AD Patient Sample Size | Mean Difference | P value | 95%-CI | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zaman (1992) [59] | 40 | N/A | 80 | 83 | 30.03 ± 12.03 | 18.65 ± 3.62 | 10 | -11.38 | p < 0.002 | -17.11;-5.65 | 5 |
| Mas (2006) [60] | 286 | 61 | 74.71 ± 10.88 | 73.52 ± 9.06 | 33.51 ± 9.56 | 32.90 ± 10.12 | 100 | -0.61 | p < 0.04 | -2.98;1.76 | 6 |
| Mangialasche (2012) [61] | 521 | 59.8 | 74.7 ± 5.3 | 77.4 ± 6.3 | 38.85 ± 5.55 | 33.04 ± 2.71 | 168 | -5.81 | p < 0.0001 | -6.7;-4.92 | 7 |
| Polidori (2004) [63] | 141 | 68.1 | 75.7 ± 7.3 | 76.8 ± 6.9 | 50.2 ± 10.2 | 37.8 ± 5.8 | 63 | -12.4 | p < 0.0001 | -15.45;-9.35 | 6 |
| Jimenez-Jimenez (1997) [64] | 81 | 44.4 | 70.1 ± 7.2 | 72.5 ± 8.6 | 31.3 ± 6.3 | 24.3 ± 8.4 | 44 | -7 | p < 0.05 | -10.21;-3.79 | 6 |
| Sinclair (1998) [65] | 83 | 47 | 73.4 ± 7.2 | 74.3 ± 8.1 | 36.0 ± 3.20 | 31.1 ± 2.90 | 25 | -4.9 | p = 0.035 | -6.4;-3.4 | 7 |
| Ciabattoni (2007) [66] | 88 | 59.1 | 75 ± 7 | 73 ± 8 | 52.0 ± 13.0 | 33 ± 15 | 44 | -19 | p = 0.059 | -24.87;-13.13 | 7 |
| Baldeiras (2008) [67] | 164 | 58.5 | 68.4 ± 1.8 | 73.0 ± 1.2 | 36.8 ± 2.2 | 28.7 ± 1.7 | 42 | -8.1 | p < 0.05 | -8.98;-7.22 | 6 |
| Raszewski (2016) [68] | 104 | 61.5 | 73.6 ± 9.4 | 77.3 ± 6.8 | 38.7 ± 7.3 | 34.2 ± 4.4 | 31 | -4.5 | p = 0.057 | -7.24;-1.76 | 8 |
| Mangialasche (2015) [62] | 90 | 51.1 | 79.1 ± 7.7 | 74.9 ± 6.9 | 46.20 ± 4.90 | 26.35 ± 4.3 | 28 | -19.85 | p < 0.001 | -22.48;-17.22 | 8 |
Table 3: Characteristics of vitamin E studies.
Primary analyses
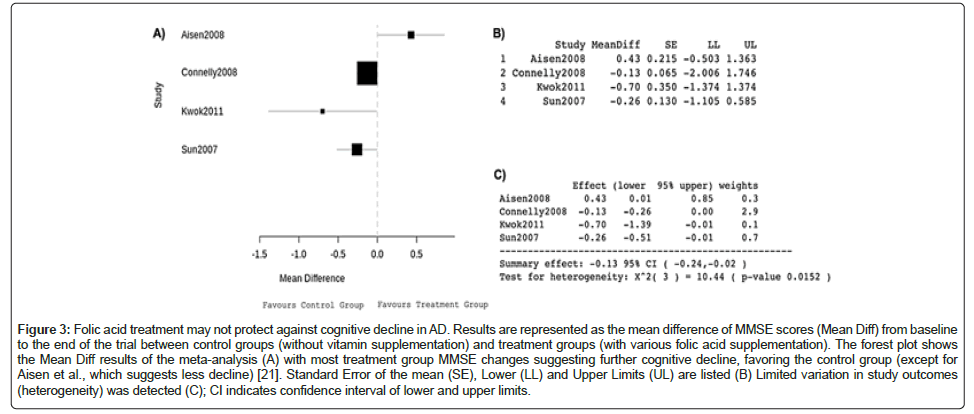
The Mini–Mental State Examination (MMSE) is a questionnaire used to measure cognitive impairment and screen for dementia, and accordingly can be used to compare AD progression between different serum vitamin patient populations. The initial folic acid data analysis compared changes in mean score on the MMSE ± SD from baseline between control groups (without vitamin supplementation) and treatment groups (with various folic acid supplementations). The mean change in MMSE score was used in the meta-analysis as a measure of cognitive function. The MMSE is out of 30 and a higher score indicates better cognition. A score between 25 and 30 is considered to be normal cognition.
The forest plot produced by the data (Figure 3A) depicts a mean MMSE score decrease after a period of at least 6 months that overall favours the non-supplemented control group. Other than Aisen et al., data from these studies suggested that folic acid supplementation correlates with increased cognitive decline as measured by the MMSE [21]. Box areas were proportional to 1/SE2, with values to 3 d.p. (Figure 3B). As SE2 represents the variance, a statistical measurement of the spread of data set numbers from the mean, larger box areas reflect less variance and greater study weight (1/SE2).
Figure 3: Folic acid treatment may not protect against cognitive decline in AD. Results are represented as the mean difference of MMSE scores (Mean Diff) from baseline to the end of the trial between control groups (without vitamin supplementation) and treatment groups (with various folic acid supplementation). The forest plot shows the Mean Diff results of the meta-analysis (A) with most treatment group MMSE changes suggesting further cognitive decline, favoring the control group (except for Aisen et al., which suggests less decline) [21]. Standard Error of the mean (SE), Lower (LL) and Upper Limits (UL) are listed (B) Limited variation in study outcomes (heterogeneity) was detected (C); CI indicates confidence interval of lower and upper limits.
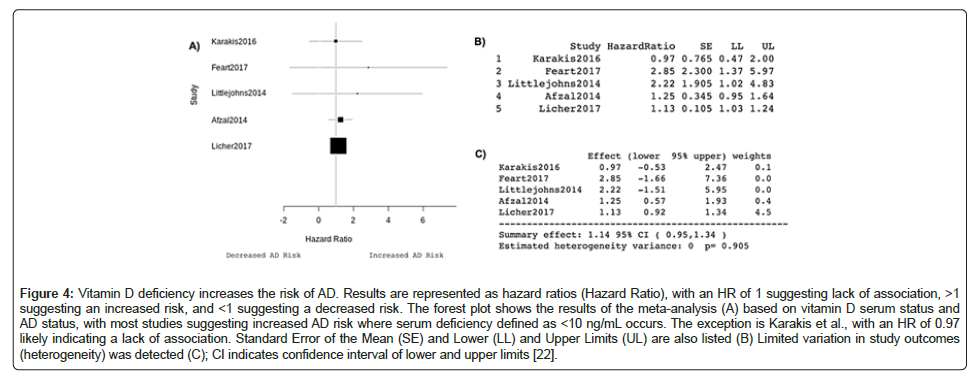
The summary effect of -0.13, 95% CI -0.24, 0.02 indicates that taking folic acid reduced the MMSE score by 0.13 (Figure 3C). There was also no significant heterogeneity following pooling of data, hence the use of a fixed-effects model of analysis. The analysis revealed a significant difference in MMSE score between the groups. However, I was not able to adjust for cohort size and sex which means despite significance, observed results may not translate to wider AD patient populations. The forest plot produced by the data (Figure 4A) illustrates how the multivariable-adjusted hazard ratios suggest that vitamin D deficiency increases the probability of AD occurrence in this specific patient population, compared to the patients with similar characteristics with adequate vitamin D. An HR greater than 1 suggests an increased risk of AD. Other than Karakis et al., which had a HR of 0.97, the included studies have HRs over 1 [22]. Box areas were proportional to 1/SE2, with values to 3 d.p. displayed in Figure 4B. The summary effect of 1.14, 95% CI 0.95,1.34 indicates that a unit decrease in vitamin D increases the risk of developing AD by 14% (Figure 4C). There was also no heterogeneity following pooling of data and a significant difference in the number of AD patients with deficiency compared to non–deficient controls.
Figure 4: Vitamin D deficiency increases the risk of AD. Results are represented as hazard ratios (Hazard Ratio), with an HR of 1 suggesting lack of association, >1 suggesting an increased risk, and <1 suggesting a decreased risk. The forest plot shows the results of the meta-analysis (A) based on vitamin D serum status and AD status, with most studies suggesting increased AD risk where serum deficiency defined as <10 ng/mL occurs. The exception is Karakis et al., with an HR of 0.97 likely indicating a lack of association. Standard Error of the Mean (SE) and Lower (LL) and Upper Limits (UL) are also listed (B) Limited variation in study outcomes (heterogeneity) was detected (C); CI indicates confidence interval of lower and upper limits [22].
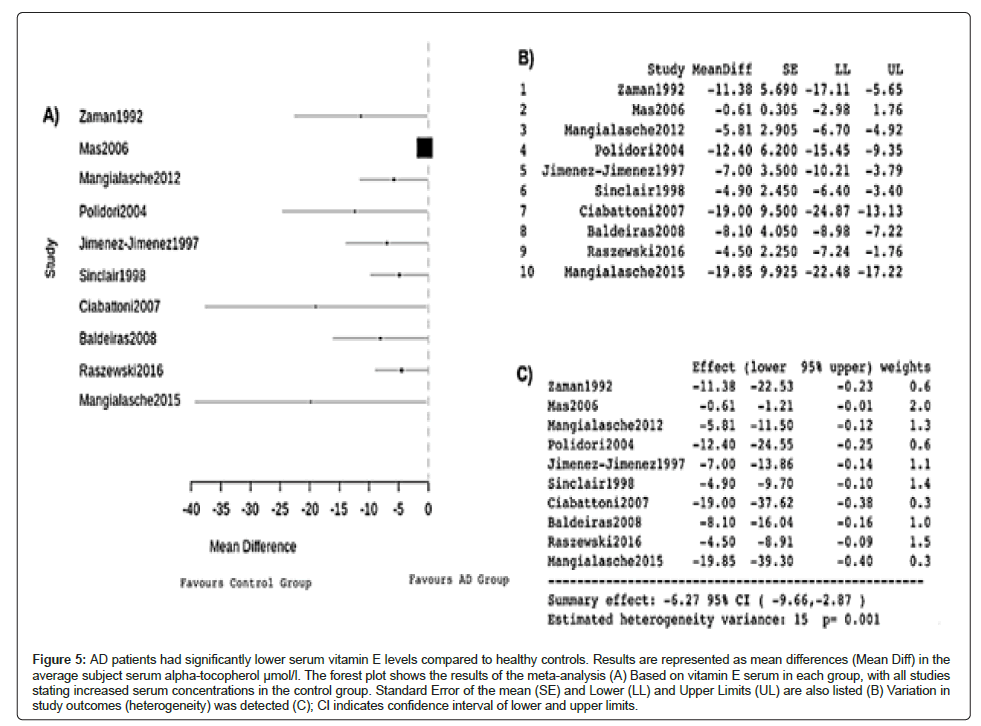
The forest plot produced by the data (Figure 5A) illustrates that the control groups of healthy patients in each study all had higher serum vitamin E than the AD groups, indicating a comparative correlation between a lower concentration of serum vitamin E and AD. The mean differences in serum vitamin E (μmol/l) were all negative, indicating higher serum vitamin E further away from deficiency in healthy patients (Figure 5B). The summary effect of -6.27, 95% CI-9.66,-2.87 further supports this relationship (Figure 5C). Significant heterogeneity was detected in this meta‐analysis, hence the use of a random-effects model, with an estimated and significant variance of 15.
Figure 5: AD patients had significantly lower serum vitamin E levels compared to healthy controls. Results are represented as mean differences (Mean Diff) in the average subject serum alpha-tocopherol μmol/l. The forest plot shows the results of the meta-analysis (A) Based on vitamin E serum in each group, with all studies stating increased serum concentrations in the control group. Standard Error of the mean (SE) and Lower (LL) and Upper Limits (UL) are also listed (B) Variation in study outcomes (heterogeneity) was detected (C); CI indicates confidence interval of lower and upper limits.
Discussion
There is considerable evidence and potential linkage that would justify the implementation of a more vitamin–oriented focus in the treatment and prevention of AD. Substantial effort has been devoted towards the development of anti-AD agents, and many of these drugs fail in clinical trials. Naturally occurring and fundamentally required vitamins’ mechanisms of action will not only directly provide patient benefit, but also be useful for guiding drug development with further data and experimentation.
It must be noted that there is a high degree of difficulty in interpreting results and identifying causality given the diversity of populations introducing a nutritional bias. Further validation of the present findings with trials of larger sample sizes and longer durations is needed. Additionally, the complexity of AD progression raises questions as to whether vitamins alone provide any impactful benefit, and so vitamin-associated molecular interactions must be studied in further detail, alongside coinciding behaviours and actions (such as exercise and sunlight relating to increased vitamin D). Given their various roles in the body and the diverse extents to which they carry this out, the preliminary results for each vitamin section provides different insight into different means to improve the prognosis of current and susceptible AD patients.
Vitamin B
The preliminary results suggest combination supplements containing folic acid (with or without vitamins B6 and/or B12) may not provide cognitive benefits over a placebo in all elderly populations with impaired cognitive function secondary to AD. Elevation of homocysteine has been linked to an increased risk of Alzheimer disease and other forms of dementia, and given the inverse association between homocysteine concentrations and cognitive performance; it is still a promising area to tackle for treatment options. In the past, vitamin B supplementation has been demonstrated to have protective effects by reducing the homocysteine levels and preserving cognition [23,24].
The efficacy of vitamin B supplementation in lowering homocysteine levels and improving cognitive AD symptoms has been shown to be predetermined by the patients’ vitamin status and baseline homocysteine levels [25], which are a factor not fully accounted for in the summary effect of the meta- analysis. Maximal effects appear to occur in those with B12 deficiency and hyperhomocysteinemia, which could imply more personalised forms of vitamin B therapy are required for optimal results. Whilst the efficiency of vitamin B supplementation in lowering homocysteine levels is unclear from the results, the mechanistic correlation with prevention of further cognitive decline is apparent in AD patients.
An additional note of caution is that the MMSE is only one part of the assessment for AD. Patient history, symptoms and a physical examination are also required to accurately judge improvements in AD on the whole rather than just the range of cognition functions assessed by the MMSE.
Connelly et al., assessed the effect of folic acid supplementation with cholinesterase inhibitors to give results that suggested isolated use of B vitamins and folic acid is ineffective in improving cognition in people with AD, despite lowered homocysteine levels [26]. In my opinion, this demonstrates that homocysteine does not necessarily solely underly the relationship between vitamin B sufficiency and improved cognitive decline, and more evaluation of this complex potential mechanism must be done (Table 1A).
Durga (2007) identified the use of daily 800 μg/d folic acid supplementation for 3 years in the improvement of various components of cognitive performance compared with placebo in older adults [24]. Fioravanti et al., prior had shown that elderly patients receiving 15mg/d folic acid for 60 days had significant improvement in memory and attention, key deficits in AD, and the intensity of improvement was positively correlated with the initial severity of folic acid deficiency, further strengthening the basis for supplementation in deficient AD patients [27]. It must also be noted that there was a weak correlation between the initial deficiency severity and the extent of cognitive decline, potentially highlighting that this aspect of AD is not heavily dependent on folic acid deficiency.
It is recognized that the initial conclusions drawn from the preliminary results have many limitations. Data were drawn from random clinical trials that compared the effect of supplementation with that of placebo and in both short-term and long-term treatment durations (Table 1B and 1C). Whilst separate analysis with the shorter-term and longer-term did not change the overall conclusion, it is notable that the long-term durations provided either a large positive or large negative disagreeing result, which may imply the effects of time heavily interact with the mental effects of supplementation (Table 1D). Another limitation is that the participants may have had different degrees or different areas of cognitive impairment, which may have developed at dissimilar rates over these varied time lengths given the intrinsic complexity of AD development, and different baseline serum homocysteine and vitamin levels. Additionally, the dosage of folic acid and B vitamin varied widely across the RCTs meaning that caution is required in interpreting the results of these studies.
Vitamin D
From the initial analysis of the vitamin D data, 25(OH)D level was inversely associated with the risk of AD suggesting supplementation and vitamin sufficiency can help to reduce elderly patients’ risk. Interestingly, the only two studies with HRs greater than 2, Feart et al., and Littlejohns et al., had the highest percentage of females with 62.3 and 69.2 respectively [28,29]. Whilst proposed mechanisms vary, it has been statistically confirmed that worldwide women are more likely to have AD than men. What’s more is that a study from Stanford University suggests a variant of the ApoE-4 gene is what causes some women to be disproportionately likely to develop AD. Given that vitamin D deficiency might pose a greater risk for ApoEɛ4 non-carrier Alzheimer's disease patients; this is worth investigating further into as it may provide a mechanistic link and thus a target for AD prevention.
Several mechanisms may explain the relationship between vitamin D and AD. Vitamin D has a neuroprotective role in the up-regulation of nerve growth factor, and brain-derived and glial cell-derived neurotrophic factors [29,30]. More directly, vitamin D promotes the removal of amyloid-β and inhibits the expression of inducible nitric oxide synthase which are heavily linked to AD pathogenesis [31-34]. Less directly but still relevant, vitamin-D-binding protein has been shown to reduce Aβ aggregation and cell death induced by Aβ, which may relate to the apparent effect in reducing AD risk [35].
Whilst the cohort studies included in the initial meta-analysis were mostly of high quality, minimizing selection bias and reverse causality, there were still various limitations of the initial analysis. Firstly, identifying changes in vitamin D levels during multiple years of follow-up may occur due to many confounding factors such as diet and sun exposure, which themselves may affect AD as opposed to simply vitamin D in the form of an oral supplement. Furthermore, the analysis participants were only of European and North American nationalities, which certainly limit the applicability of results to the wider, ideal population of elderly and AD susceptible people worldwide. Also, there remains the issue of reverse causation given that older people already tend to have lower serum vitamin D, and as AD onset causes neglect, dietary changes and reduced outdoor activity, which in turn result in lower concentrations [36].
Finally, variations in the diagnostic criteria of Alzheimer's disease between studies may lead to some bias and inaccuracy in conclusions, including errors in distinguishing different types of dementia resulting in the attenuation of the true association between vitamin D and AD.
Vitamin E
The antioxidant function of vitamin E seems like the most likely principal mechanism underlying the association between serum vitamin E and AD. Even with consideration of the complexity of identifying the cause of AD, the involvement of oxidative stress resulting from increased content of reactive nitrogen or oxygen species has been widely recognized. As well as this, other mechanisms beyond antioxidant function have been explored. This includes increased focus on how vitamin E affects membrane fluidity, signaling, and gene regulation [36], with the notable example of vitamin E restraining protein kinase C activation by preventing its phosphorylation [37], which is important as PKC is implicated in controlling memory and learning alongside regulation of signaling pathways involved in amyloid and tau pathologies [38].
Further still, it was demonstrated that vitamin E strongly affects the expression of various mammalian genes encoding for proteins involved in the clearance of amyloid β-protein [39]. This displayed protective role of vitamin E in AD progression closely matches the correlation revealed by the initial vitamin E meta-analysis, though of course only being demonstrated in an animal model thus far limits conclusions and applications that can be drawn from it [40-42]. The comprehensive mechanisms underlying the association between vitamin E and AD, in the future, would be elucidated by conducting further human experiments. Whilst proposed mechanisms vary and remain uncertain, the discoveries made from the preliminary results hold important clinical value [43-46]. Vitamin E deficiency may be an important component in the prognosis and subsequent treatment options for such AD patients, with the potential of predicting mild cognitive impairment progression to AD.
This initial analysis also had its own limitations that require improvement upon. The included studies were performed in Europe, most likely due to its higher AD incidence, but at the expense of results not being completely representative of a wider ethnic and cultural population. Additionally, not all outcomes in the included studies were of adjusting confounders causing some inaccuracies in the results. Finally, to ideally determine a causal relationship between serum vitamin E and AD in elderly people, an extensive prospective cohort study is required [46-68].
Conclusion
The proposal is to systematically investigate vitamin levels in AD patients and identify any association with poor MMSE scores. A series of randomized, double-blind, placebo controlled trials can then be conducted based on these results to identify patients with good responses after 24 months of treatment, defined by NICE criteria (NICE, 2001) as improvement or no deterioration in MMSE score, alongside evidence of global improvement on the basis of behavioral and/or functional assessment.
The main objective is to reveal an association between vitamin levels and cognitive status (and decline) in AD patients. Whether the association is positive or negative, this will greatly aid developments in the approach to AD treatment important to patients, health professionals and policy makers. Objectives also include: determining whether deficient vitamin concentrations are associated with more drastic cognitive decline; the possibility of improving cognitive status in deficient and sufficient patients through supplementation; assessing the effect of supplements on AD progression based on physical Activities of Daily Living (ADLs). The various aforementioned limitations of the preliminary results can be used to improve the quality of the research experiment, with wider scale aims of adjusting the approach of individuals and healthcare systems to AD treatment. As well as direct supplementation, behavioral changes including diet, clothing and lifestyle choices that influence serum vitamin levels may be encouraged to be altered as a result of more concrete evidence.
The proposed hypothesis is that serum vitamin B, D, and E levels and status (sufficient or deficient) contribute towards AD development and progression in elderly patients. Accordingly, supplementation may aid in the improvement of AD symptoms as measured by cognitive status through MMSEs. Based on the preliminary results, vitamin B deficiency can coincide with elevated homocysteine and worsening of AD symptoms; vitamin D deficiency increases the risk of AD; and vitamin E deficiency increases the risk of AD and aids its progression through susceptibility to enhanced oxidative damage. Accordingly, sufficiency in these respective vitamins acts to prevent AD and/or slow its progression in patients, with noticeable improvement in cognitive symptoms.
Whilst the initial meta-analysis provided a clearer understanding of correlations and potential mechanisms, the many mentioned limitations prevent robust clinical decisions being made with these information clinical changes with the potential to improve millions of present and future patients’ qualities of life. A more thorough investigation is needed. The programme of research must ultimately investigate if serum vitamin levels affect AD progression and risk. Specifically, deficiency in vitamins B, D, and E being related to increased AD risk and progression as measured by cognition via MMSE scores.
Standard medical resources must also be available to enable physical examination findings, including changes in vital signs, laboratory test abnormalities, and concomitant medication use. Patient caregivers are also required to ensure compliance of study medication and monitoring of adverse events throughout the study.
In recent years, increasing evidence suggests modifiable risk factors play a more significant role in the pathogenesis of AD than previously thought, and so modifying these factors as an alternative approach to delay or prevent risk of this disease will benefit a wide range of patient populations. Patients with increased risk due to genetic conditions and/or a family history of AD will benefit from minimising their risk through vitamin supplementation if such an association is discovered. As an example, the aforementioned prevalence of the ApoE-4 gene gives this group the possibility to greatly benefit from mitigation of AD risk, especially beneficial where early onset is an issue. Additionally, women (with and without genetic variants) naturally have a higher risk of AD, and given the more common late age of onset, women over 65 years old will be a key beneficiary.
Ethnically, Asian, Hispanic, Black and Ashkenazi Jew populations are placed at a higher risk of AD and so may also benefit from research findings. Patients with conditions which affect vitamin intake on a metabolic level (including renal and hepatic disease) as well as those who suffer from neurological disorders such as neglect syndrome will also be likely to have deficiencies in a plethora of vitamins, and accordingly will benefit from this research. This is especially important where vitamin D is concerned for elderly patients, as thinner skin due to ageing decreases its synthesis efficiency, impaired absorption of nutrients occurs, increased time indoors and minimal exposure to natural sunlight further put them at risk and thus more likely to benefit from this research. Despite efforts in understanding, preventing and treating its complex pathogenesis, AD is also a major challenge in public health economically. Affordable treatment and risk reduction in the form of vitamin supplementation will directly benefit healthcare systems and further indirectly AD patients by freeing more resources to be used. There are many ethical issues of the clinical trial aspect of this research, however. Firstly, the issue of ‘therapeutic misconception’ the misapprehension patients may have that what is being offered is certainly therapeutic. As the trial is for investigative purposes, this can give false hope to the treated, supplemented patients. The use of a placebo in itself may be regarded as unethical and deceptive, as patients on the placebo arm of a clinical trial must be made to believe they are receiving a working treatment for the placebo effect to play a role at all. This raises the issue of ‘informed consent’, where patients must be provided with all the information, they need to make a voluntary decision about study participation; this decision is open to misunderstandings by nature of the double–blinded and placebo control trial. To avoid the possibility of placebo participants being harmed by missing active treatment however, standard AD treatments are allowed insofar as they do not significantly alter vitamin serum levels. Additionally, treatment group patients themselves are at risk of harm if the vitamin supplements are intolerable or cause adverse effects such as enhanced mental decline.
I aim to communicate this research with potential beneficiaries through local healthcare systems including information distributed by pharmacies and hospitals, as well as through medical journals and more general news platforms to ensure the research reaches as many people as possible.
Ethics Statement
Ethical approval was not necessary in this meta–analysis given that all included data was based on pre-existing and previously published studies.
References
- Burns A, Iliffe S (2009) Alzheimer's disease. Bmj 338.
[Crossref] [Google Scholar] [PubMed]
- Godswill AG, Somtochukwu IV, Ikechukwu AO, Kate EC (2020) Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review. Int J Food Sci 3(1):1-32.
- Morris MS (2003) Homocysteine and Alzheimer's disease. Lancet Neurol 2(7):425-8.
[Crossref] [Google Scholar] [PubMed]
- Flirski M, Sobow T (2005) Biochemical markers and risk factors of Alzheimer's disease. Curr Alzheimer Res. 2(1):47-64.
[Crossref] [Google Scholar] [PubMed]
- Douaud G, Refsum H, de Jager CA, Jacoby R, E. Nichols T, et al. (2013) Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci 110(23):9523-8.
[Crossref] [Google Scholar] [PubMed]
- Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, et al. (2009) 1α, 25-Dihydroxyvitamin D 3 interacts with curcuminoids to stimulate amyloid-β clearance by macrophages of Alzheimer's disease patients. J Alzheimer's Dis 17(3):703-17.
[Crossref] [Google Scholar] [PubMed]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat 29(1):21-30.
[Crossref] [Google Scholar] [PubMed]
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, et al. (2014) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83(10):920-8.
[Crossref] [Google Scholar] [PubMed]
- E Abdel Moneim A (2015) Oxidant/antioxidant imbalance and the risk of Alzheimer's disease. Curr Alzheimer Res 12(4):335-49.
[Crossref] [Google Scholar] [PubMed]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, et al. (2013) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med 336(17):1216-22.
[Crossref] [Google Scholar] [PubMed]
- Peterson J, Welch V, Losos M, Tugwell PJ (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute 2(1):1-2.
- Team RC (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Schwarzer G. Metasens: Brief overview of methods and general hints.
- van Rossum G, Drake Jr FL (1995) Python tutorial. The Netherlands: Centrum voor Wiskunde en Informatica.
- Borenstein M, Hedges L, Rothstein H (2007) Meta-analysis: Fixed effect vs. random effects. Meta-analysis. com.
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315(7109):629-34.
[Crossref] [Google Scholar] [PubMed]
- Shields PG (2000) Publication bias is a scientific problem with adverse ethical outcomes: The case for a section for null results. Cancer Epidemiol Biomarkers Prev. 9(8):771-2.
[Google Scholar] [PubMed]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 346(7):476-83.
[Crossref] [Google Scholar] [PubMed]
- McCaddon A, Hudson P, Davies G, Hughes A, Williams JH, et al. (2001) Homocysteine and cognitive decline in healthy elderly. Dement Geriatr Cogn Disord 12(5):309-13.
[Crossref] [Google Scholar] [PubMed]
- de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD (2012) Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int J Geriatr Psychiatry 27(6):592-600.
[Crossref] [Google Scholar] [PubMed]
- Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, et al. (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. Jama 300(15):1774-83.
[Crossref] [Google Scholar] [PubMed]
- Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, et al. (2016) Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimer's Dis 51(2):451-61.
[Crossref] [Google Scholar] [PubMed]
- Connelly PJ, Prentice NP, Cousland G, Bonham J (2008) A randomised double blind placebo controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's disease. Int J Geriatr 23(2):155-60.
[Crossref] [Google Scholar] [PubMed]
- Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, et al. (2007) Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. The Lancet 369(9557):208-16.
[Crossref] [Google Scholar] [PubMed]
- Dursun E, Alaylıoglu M, Bilgic B, Hanagası H, Lohmann E, et al. (2016) Vitamin D deficiency might pose a greater risk for ApoEɛ4 non-carrier Alzheimer’s disease patients. Neurol Sci 37(10):1633-43.
[Crossref] [Google Scholar] [PubMed]
- Connelly PJ, Prentice NP, Cousland G, Bonham J (2008) A randomised double blind placebo controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's disease. Int J Geriatr 23(2):155-60.
[Crossref] [Google Scholar] [PubMed]
- Fioravanti M, Ferrario E, Massaia M, Cappa G, Rivolta G, et al. (1997) Low folate levels in the cognitive decline of elderly patients and the efficacy of folate as a treatment for improving memory deficits. Arch Gerontol Geriatr 26(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, et al. (2017) Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement 13(11):1207-16.
[Crossref] [Google Scholar] [PubMed]
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, et al. (204) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83(10):920-8.
[Crossref] [Google Scholar] [PubMed]
- Flicker L, Vasikaran SD, Thomas J, Acres JM, Norman P (2006) Efficacy of B vitamins in lowering homocysteine in older men: Maximal effects for those with B12 deficiency and hyperhomocysteinemia. Stroke 37(2):547-9.
[Crossref] [Google Scholar] [PubMed]
- Naveilhan P, Neveu I, Baudet C, Funakoshi H, Wion D, et al. (1996) 1, 25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Mol Brain Res 41(1-2):259-68.
[Crossref] [Google Scholar] [PubMed]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D (2002) New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 13(3):100-5.
[Crossref] [Google Scholar] [PubMed]
- Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, et al. (2009) 1α, 25-Dihydroxyvitamin D 3 interacts with curcuminoids to stimulate amyloid-β clearance by macrophages of Alzheimer's disease patients. J Alzheimer's Dis 17(3):703-17.
[Crossref] [Google Scholar] [PubMed]
- Mizwicki MT, Menegaz D, Zhang J, Barrientos-Duran A, Tse S, et al. (2012) Genomic and nongenomic signaling induced by 1α, 25 (OH) 2-vitamin D 3 promotes the recovery of amyloid-β phagocytosis by Alzheimer's disease macrophages. J Alzheimer's Dis 29(1):51-62.
[Crossref] [Google Scholar] [PubMed]
- Dursun E, Gezen-Ak D, Yilmazer S (2013) A new mechanism for amyloid-β induction of iNOS: Vitamin D-VDR pathway disruption. J Alzheimer's Dis 36(3):459-74.
[Crossref] [Google Scholar] [PubMed]
- Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B (2002) Inducible nitric oxide synthase and argininosuccinate synthetase: Co-induction in brain tissue of patients with Alzheimer's dementia and following stimulation with β-amyloid 1–42 in vitro. Neurosci Lett 322(2):121-5.
[Crossref] [Google Scholar] [PubMed]
- Moon M, Song H, Hong HJ, Nam DW, Cha MY (2013) Vitamin D-binding protein interacts with Aβ and suppresses Aβ-mediated pathology. Cell Death Differ 20(4):630-8.
[Crossref] [Google Scholar] [PubMed]
- Brigelius-Flohe R (2009) Vitamin E: The shrew waiting to be tamed. Free Radic Biol Med 46(5):543-54.
- Ricciarelli R, Tasinato A, Clement S, Ozer Nk, Boscoboinik D, et al. (1998) α-Tocopherol specifically inactivates cellular protein kinase C α by changing its phosphorylation state. Biochem J 334(1):243-9.
[Crossref] [Google Scholar] [PubMed]
- Talman V, Pascale A, Jantti M, Amadio M, Tuominen RK (2016) Protein Kinase C Activation as a Potential Therapeutic Strategy in Alzheimer's Disease: Is there a Role for Embryonic Lethal Abnormal Vision‐like Proteins? Basic Clin Pharmacol Toxicol 119(2):149-60.
[Crossref] [Google Scholar] [PubMed]
- Rota C, Rimbach G, Minihane AM, Stoecklin E, Barella L (2005) Dietary vitamin E modulates differential gene expression in the rat hippocampus: potential implications for its neuroprotective properties. Nutr Neurosci 8(1):21-9.
[Crossref] [Google Scholar] [PubMed]
- Boucher BJ (2012) The problems of vitamin d insufficiency in older people. Aging Dis 3(4):313.
[Google Scholar] [PubMed]
- Edwards III GA, Gamez N, Escobedo Jr G, Calderon O, Moreno-Gonzalez I (2019) Modifiable risk factors for Alzheimer’s disease. Front Aging Neurosci 146.
- Rakesh G, Szabo ST, Alexopoulos GS, Zannas AS (2017) Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis 8(8-9):121-36.
[Crossref] [Google Scholar] [PubMed]
- Vina J, Lloret A (2010) Why women have more Alzheimer's disease than men: Gender and mitochondrial toxicity of amyloid-β peptide. J Alzheimer's Dis 20(s2):S527-33.
- Anderson NB, Bulatao RA, Cohen B, Race P (2004) Ethnic differences in dementia and Alzheimer's disease. InCritical perspectives on racial and ethnic differences in health in late life. National Academies Press (US).
- Truswell D (2013) Black, Asian and Minority Ethnic Communities and Dementia. Better Health Briefing.
- Meehan M, Penckofer S (2014) The role of vitamin D in the aging adult. J Aging Gerontol 2(2):60-71.
[Crossref] [Google Scholar] [PubMed]
- Raman M, Milestone AN, Walters JR, Hart AL, Ghosh S (2011) Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol 4(1):49-62.
[Crossref] [Google Scholar] [PubMed]
- Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, et al. (2015) Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol 72(11):1295-303.
[Crossref] [Google Scholar] [PubMed]
- Kwok T, Lee J, Law CB, Pan PC, Yung CY, et al. (2011) A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clin Nutr 30(3):297-302.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC (2007) Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: A 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther 29(10):2204-14.
[Crossref] [Google Scholar] [PubMed]
- Blass JP, Gleason P, Brush D, DiPonte P, Thaler H (1988) Thiamine and Alzheimer's disease: A pilot study. Arch Neurol 45(8):833-5.
[Crossref] [Google Scholar] [PubMed]
- Birkmayer JG (1996) Coenzyme nicotinamide adenine dinucleotide: new therapeutic approach for improving dementia of the Alzheimer type. Ann Clin Lab Sci 26(1):1-9.
[Google Scholar] [PubMed]
- Phelan MJ, Mulnard RA, Gillen DL, Schreiber SS (2017) Phase II clinical trial of nicotinamide for the treatment of mild to moderate Alzheimer’s disease. J Geriatr Med Gerontol 3(2469):10-23937.
- Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, et al. (2017) Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement 13(11):1207-16.
[Crossref] [Google Scholar] [PubMed]
- Afzal S, Bojesen SE, Nordestgaard BG (2014) Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 10(3):296-302.
[Crossref] [Google Scholar] [PubMed]
- Licher S, de Bruijn RF, Wolters FJ, Zillikens MC, Ikram MA, et al. (2017) Vitamin D and the risk of dementia: the Rotterdam study. J Alzheimer's Dis 60(3):989-97.
[Crossref] [Google Scholar] [PubMed]
- Zaman Z, Roche S, Fielden P, Frost PG, Niriella DC, et al. (1992) Plasma concentrations of vitamins A and E and carotenoids in Alzheimer's disease. Age and ageing 21(2):91-4.
[Crossref] [Google Scholar] [PubMed]
- Mas E, Dupuy AM, Artero S, Portet F, Cristol JP, et al. (2006) Functional Vitamin E deficiency in ApoE4 patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 21(3):198-204.
[Crossref] [Google Scholar] [PubMed]
- Mangialasche F, Xu W, Kivipelto M, Costanzi E, Ercolani S, et al. (2012) Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging 33(10):2282-90.
[Crossref] [Google Scholar] [PubMed]
- Mangialasche F, Baglioni M, Cecchetti R, Kivipelto M, Ruggiero C, et al. (2015) Lymphocytic mitochondrial aconitase activity is reduced in Alzheimer's disease and mild cognitive impairment. J Alzheimer's Dis 44(2):649-60.
[Crossref] [Google Scholar] [PubMed]
- Polidori MC, Mattioli P, Aldred S, Cecchetti R, Stahl W, et al. (2004) Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: Relevance to Alzheimer disease and vascular dementia. Dement Geriatr Cogn Disord 18(3-4):265-70.
[Crossref] [Google Scholar] [PubMed]
- Jimenez-Jimenez FJ, de Bustos F, Molina JA, Benito-Leon J, Tallon-Barranco A, et al. (1997) Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Alzheimer's disease. J Neural Transm 104(6):703-10.
[Crossref] [Google Scholar] [PubMed]
- Sinclair AJ, Bayer AJ, Johnston JO, Warner C, Maxwell SR (1998) Altered plasma antioxidant status in subjects with Alzheimer's disease and vascular dementia. Int J Geriatr Psychiatry 13(12):840-5.
[Crossref] [Google Scholar] [PubMed]
- Ciabattoni G, Porreca E, Di Febbo C, Di Iorio A, Paganelli R, et al. (2007) Determinants of platelet activation in Alzheimer's disease. Neurobiol Aging 28(3):336-42.
[Crossref] [Google Scholar] [PubMed]
- Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, et al. (2008) Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer's disease. J Alzheimer's Dis 15(1):117-28.
[Crossref] [Google Scholar] [PubMed]
- Raszewski G, Chwedorowicz R, Chwedorowicz A, Rothenberg KG (2016) Homocysteine, antioxidant vitamins and lipids as biomarkers of neurodegeneration in Alzheimer’s disease versus non-Alzheimer’s dementia. Ann Agric Environ Med 23(1).
[Crossref] [Google Scholar] [PubMed]
Citation: Akande R (2022) A Meta-Analysis on the Association of Vitamin B, D, and E Levels with the Progression and Risk of Alzheimer's Disease. J Alzheimers Dis Parkinsonism 12: 555. DOI: 10.4172/2161-0460.1000555
Copyright: © 2022 Akande R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.