Research Article Open Access
A Manual, Semi-Automated and Automated ROI Study of fMRI Hemodynamic Response in the Caudate Nucleus
| Jing Zhang1*, Erin A Hazlett2, King-Wai Chu2 and Monte S Buchsbaum2 | |
| 1Department of Bioinformatics, School of Biomedical Engineering, Capital Medical University, Beijing 100069, PR China | |
| 2Department of Psychiatry, Mount Sinai School of Medicine, New York, NY 10029, USA | |
| Corresponding Author : | Jing Zhang School of Biomedical Engineering Capital Medical University Beijing 100069, PR China Tel: +86-10-8391-1363 Fax: +86-10-8391-1544 E-mail: jzhang0000@163.com |
| Received September 15, 2013; Accepted October 15, 2013; Published October 20, 2013 | |
| Citation: Zhang J, Hazlett EA, Chu KW, Buchsbaum MS (2013) A Manual, Semi-Automated and Automated ROI Study of fMRI Hemodynamic Response in the Caudate Nucleus. OMICS J Radiology 2:150. doi: 10.4172/2167-7964.1000150 | |
| Copyright: © 2013 Zhang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
This study investigated abnormalities of fMRI Hemodynamic Response (HR) in the caudate nucleus in schizophrenia with manual, semi-automated and automated approaches. The three approaches were applied to generate the Region of Interest (ROI) and extract fMRI HR from the ROI. Compared with controls, less activation with delayed fMRI HR in the caudate were observed in the patient group. High correlations of the AUC were found between the manual and semiautomated ROI approaches, but not between the manual and the automated ROI approach. In addition, the location and size of the automated box ROI are critical for the fMRI HR curve extracted. The abnormal fMRI hemodynamic response in the caudate in the patient group may suggest functional deficits in the frontal-striatal-thalamic circuit in schizophrenia. To speed up fMRI data analysis in anatomical ROI, the semi-automated approach may be used as an alternative to the manual approach in detecting fMRI experimental effect.
| Keywords |
| Regions of interest; fMRI; Hemodynamic response; Area under the curve |
| Introduction |
| Since Blood-Oxygen-Level Dependent (BOLD) Functional Magnetic Resonance Imaging (fMRI) was introduced [1], BOLD fMRI has been widely used to investigate the human brain in vivo by measuring regional cerebral blood flow and revealing the underlying neural activity. Recent advances in fMRI allow researchers to study psychiatric disorders with better spatial and temporal resolutions. Consequently, there is growing interests in applying fMRI to psychiatric research [2-9]. fMRI and other functional neuroimaging techniques have demonstrated that resting neural activity and activation during a variety of cognitive tasks are abnormal in schizophrenia [10] in brain regions such as the prefrontal and temporal cortex, cingulate gyrus, hippocampus, striatum, thalamus and the cerebellum [5,11]. Reduced and delayed hemodynamic responses in schizophrenia has been detected by fMRI [11,12] and there is also evidence that people genetically at risk of schizophrenia have changed spatial patterns of brain activity in the face of apparently normal cognition [13,14]. Furthermore, Whalley et al. [15] reported that fMRI technique may identify people in whom the first symptoms are beginning to emerge [14] which suggest that early treatment may be important. |
| The caudate nucleus is a sub-cortical region in the striatum that plays an important role in voluntary movement control, memory and learning. It is linked to the frontal cortex and the thalamus through the frontal-striatal-thalamic circuit. Compared with controls, reduced activations in the caudate nucleus in schizophrenia have been reported by a number of fMRI studies in tasks such as prepulse inhibition startle, working memory and learning [4,12,15,16]. Previous studies also indicate that striatal abnormalities occurred in schizophrenia patients and unaffected siblings [17]. |
| In order to detect regional BOLD signal changes in the brain, fMRI time course is usually extracted from Regions of Interest (ROIs). One common fMRI ROI analysis is to create small ROIs at the peaks of activation clusters. Another approach is to specify a set of anatomical ROIs (regardless of activation or not) and perform statistical analysis on the fMRI data across these regions [18]. Manual delineation of ROIs is relatively accurate for ROIs such as sub-cortical structures, but manually tracing ROI is time consuming, hard for large sample study and often lack of reproducibility across different tracers or laboratories. In practice, since there can be substantial variability between individuals in anatomy, it requires caution whether the ROI analysis is based on single-subject anatomical atlas or the Talairach atlas [18]. In order to minimize manual intervention, some researchers have suggested other analysis methods such as using automated program to label brain regions [19,20]. These automated methods are simple and quick, but limited by the potential inaccuracy introduced by spatial normalization to a brain template. An alternative is semi-automated approach which combines manual and automatic approaches, e.g., SABRE [21] is based on user defined landmarks and regions of interest defined by Dade et al. [22]. In addition, semiautomated tracing software such as SNAP can speed up the tracing of ROIs [23]. |
| In this study, we considered the caudate nucleus as the ROI and investigated the fMRI hemodynamic response in the caudate nucleus in schizophrenia with 3 approaches: (1) manual delineation of the ROI, (2) semi-automated delineation that traced the caudate with the SNAP program on the MNI (Montreal Neurological Institute) brain, and (3) automated delineation of the ROI. |
| Materials and Methods |
| fMRI data |
| The fMRI data were acquired from a startle study with 13 unmedicated schizophrenia patients (3 females, 10 males; mean+SD age: 38.5+15.9 years) and 13 healthy controls (5 females, 8 males; mean ± SD age: 35.9 ± 11.7 years) whom were scanned on a head- dedicated Siemens Allegra 3.0-Tesla MRI scanner at the Mount Sinai Medical Center. This study was approved by the IRB at the Mount Sinai School of Medicine. There was no significant difference in age and sex between the patient and control groups. The fMRI acquisition occurred during an event-related attention-to-prepulse paradigm where the major stimuli were the attended and ignored tones followed by a startle sound. Details of the paradigm are described by Volz et al. [16]. The BOLD imaging was performed using a gradient echo planar (GE-EPI) sequence (28 axial slices, 3 mm thick, skip=1 mm, TR=2s, TE=40 ms, flip angle=90°, FOV=210, matrix=64×64) and the participants underwent six 4.5-min BOLD fMRI scan blocks. |
| For structural images, a T1-weighted MP-RAGE (Magnetization Prepared Rapid Gradient Echo) was used (208 slices with slice thickness=0.82 mm, matrix size=256×256×208, FOV=21 CM, TR=2500 ms, TE=4.38 ms, TI=1100 ms and an 8° flip angle FLASH acquisition). |
| Data processing |
| The following pre-processing was performed on the fMRI data with tools provided by FSL software [24]. Motion correction with FSL.MCFLIRT; brain extraction with FSL.BET; mean-based intensity normalization and high-pass temporal filtering (FSL temporal filter, sigma=100.0s). |
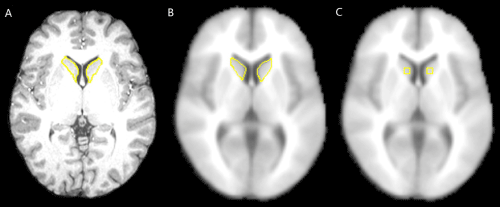
| fMRI ROI analysis were performed in 3 ways: (1) manual ROI approach: caudate ROI was traced on anterior-posterior commissure (ACPC)-positioned individual MRI and applied to the coregistered fMRI images (Figure 1A); (2) semi-automated ROI approach: ROI was traced on the MNI (Montreal Neurological Institute) template with the SNAP program (a semi-automated tracing tool) and applied to each subject’s fMRI image that was normalized to the MNI (Montreal Neurological Institute) brain template (Figure 1B); and (3) automated ROI approach: fMRI data were normalized to MNI brain template and stereotactic box-shape ROIs were specified with Talairach coordinates (Figure 1C). |
| To understand the impact of location and size of the automated ROI, pairs of box-based ROIs were placed on the caudate: with center (12, 12, 12), (16, 12, 12), (16, 16, 12), (12, 8, 12) (in Talairach coordinates) for the right caudate; and (-12, 12, 12), (-16, 12, 12), (-16, 16, 12), (-12, 8, 12) for the left caudate respectively. For simplicity, the 4 pairs of box-based ROIs are addressed as x12y12, x16y12, x16y16 and x12y8. Three sizes (3×3×3, 5×5×5, 7×7×7) of these box-shape ROIs were defined with Talairach coordinates to see the impact of automated ROI size. The box-based ROIs were automatically generated by the software developed in the Neuroscience PET Laboratory at the Mt. Sinai Medical Center. |
| The details on how hand-traced ROIs were generated were described in fMRI study [16]. Briefly, the ROIs were traced on the structural MRI and applied to the co-registered fMRI data. The fMRI hemodynamic response time course extracted from these ROIs were averaged over all voxels within the ROIs across all trials. |
| Statistical analysis |
| Analysis of Variance (ANOVA) was performed on the time course data extracted from the ROIs. The set up of mixed-design ANOVA was: Group×Condition (attended tone, ignored tone)×Time. Multivariate Wilks and Greenhouse-Geisser epsilon corrections were used to adjust repeated-measures F values on the mean of each ROI. Hemodynamic response curves were drawn under each condition. |
| The Area Under the Curve (AUC) of hemodynamic response was used to measure the performance of different ROIs. AUC was calculated in 4 ways: (1) adding only positive points in the BOLD response curve; (2) adding the root mean square of the points in the curve; (3) adding all points in the curve; (4) adding the absolute values of points in the curve. |
| Correlations between box-based ROI and hand-traced ROI were computed and t-test between patients and controls was performed on the AUC results. |
| Results |
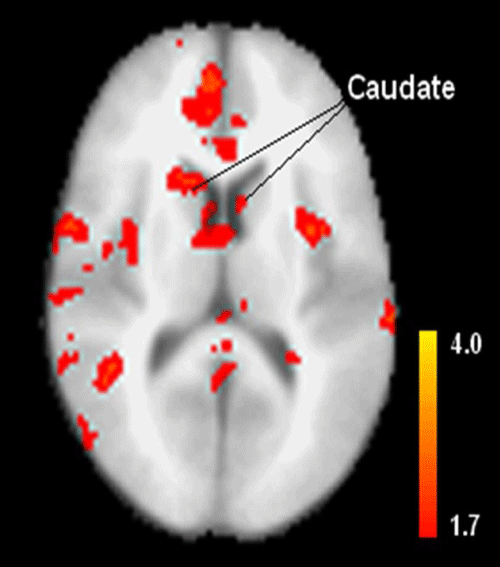
| The Statistical Parametric Map (SPM) in Figure 2 is a comparison between patients and controls. It reveals that schizophrenia patients had less activation in the caudate than controls, which is partially reflected in Table 1. |
| When comparing the two groups with the Area Under the Curve (AUC) measures, Table 2 indicates that patients have smaller AUC than controls using the manual (significant at 1-tailed t-test, p=0.091), semi-automated (not significant, p=0.123), and automated ROI (with size 3×3×3, 5×5×5 and 7×7×7 boxes centered at (± 12,12,12), significant at 1-tailed t-test, p=0.077, 0.063 and 0.053) approaches measured by Root Mean Square (RMS). The results of effect size are consistent with t-test results (Table 2). |
| The correlations between manual and semi-automated, manual and automated ROI approaches (included size 3×3×3, 5×5×5 and 7×7×7 boxes) are listed in Table 3. One can see that the correlation of area under the hemodynamic response curve (AUC) between manual and semi-automated approaches is significantly high (R=0.81-0.96), while the correlations of AUC between manual and automated ROI approaches are relatively low. Among the 4 box ROIs compared (size 3×3×4, 5×5×5 and 7×7×7 voxel-boxes are at the same z level, and size 7×7×7 voxel-box is at another z level), the box with size 7×7×7 voxels centered at ( ± 12, 12, 12) has the highest correlation with the manual ROI which indicates that a larger automated box ROI when placed properly can be better correlated with the manual tracing. |
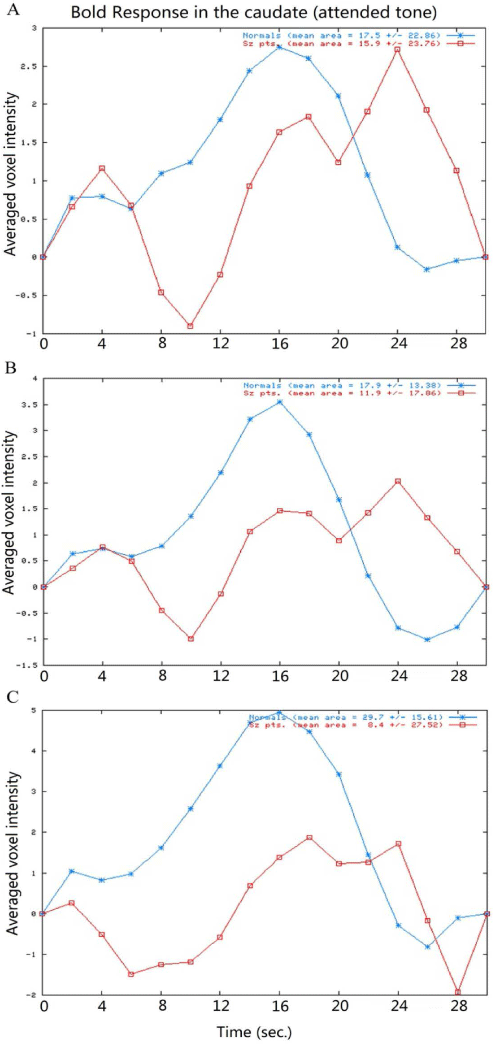
| The comparison of the hemodynamic response time course of the manual, semi-automated and automated ROI approaches shows that similar pattern of reduced and delayed hemodynamic responses in the caudate in the patients compared with the controls (Figure 3). The hemodynamic response time course extracted from the automated box ROIs indicates that (1) The shapes and amplitudes of the hemodynamic response curves extracted from the box-based ROIs are different between the left and right hemisphere (higher in right hemisphere); (2) The hemodynamic response curves vary from one ROI location to another which suggests that the location of the ROI box is critical to such automated ROI analysis (Figure 4). |
| Discussion |
| In this study, we compared the manual, semi-automated and automated ROI methods in extracting fMRI hemodynamic response time course in the caudate for schizophrenia patients and normal controls. We found that (1) schizophrenia patients have less activation with reduced hemodynamic response compared with controls (across the three ROI approaches); (2) The area under the hemodynamic response curves (AUCs) obtained from the manual and semi-automated approaches were highly correlated while large size automated box with appropriate location could generate AUC well-correlated with that of the manual approach. (3) The location and size of the automated box ROI is critical for the fMRI hemodynamic response curves extracted. |
| The first finding (reduced activations in the caudate nucleus in schizophrenia) is consistent with the findings in other fMRI studies in schizophrenia with tasks such as working memory and learning [4,12,15]. Since the structural and functional connectivity of various brain regions facilities brain function integration, activated brain regions with certain activation patterns may reflect the underlying functional neural networks [25]. Since the caudate is linked with the frontal-striatal-thalamic circuitry, abnormal hemodynamic response in the caudate in patients with schizophrenia may reflect the functional deficits (e.g., attention impairment) in their frontal-striatal-thalamic circuitry. Such findings have been reported and further discussed by Hazlett et al. [17,26]. |
| The rest of the findings in this study are related to the 3 ROI methods and all of them are based on anatomical ROIs. There are arguments on the weakness and strengths of anatomical ROIs vs. Functional ROIs (fROIs) in fMRI studies. In a pharmacological fMRI study [25,26], compared the anatomical and fROIs and found that the anatomical ROI (combined with an index of top 20% voxels of activation) was more reliable than the fROI approach in detecting the experimental effect [26]. In addition, they concluded that fROIs should be used with caution because the use of fROIs from individual sessions introduced unacceptable biases in the results, while the use of union fROIs yielded a lower sensitivity than anatomical ROIs [26,27]. However, when studying resting-state fMRI with functional connectivity measures and introducing a data-driven method for generating an ROI atlas by parcellating whole brain resting-state fMRI data into spatially coherent regions of homogeneous functional connectivity, Craddock et al. [28] found that the evaluated anatomical atlases showed poor ROI homogeneity which failed to reproduce functional connectivity results accurately [27]. These studies indicate that it may be appropriate to use anatomical ROIs for fMRI studies on hemodynamic response and activation, and use fROIs for studies on functional connectivity. |
| Despite the obvious differences in shape, size and different registration space between the manual and semi-automated ROI approaches, the two methods had significantly high (p<0.05) correlations, and detected smaller AUC of fMRI hemodynamic response in schizophrenia patients than controls (with RMS measurement), which suggests that the two methods extract similar time course from the fMRI data. However, there was relatively low correlation in the AUC between hand-traced ROI and box-based ROI (x12y12) with size 7×7×7 voxels and significant group difference for the box-based ROI. This can be explained by comparing the ROI locations in Figure 1 and the activated regions in the caudate in Figure 2 (SPM with contrast for effect of control - patient at group level). Figure 2 reveals that the differences in BOLD activations between control and patient groups are not uniformly distributed within the caudate which may be caused by the non-uniform BOLD signal distribution in the caudate of both groups. Since the box-based ROIs are located in the caudate regions which cover more significantly activated voxels, while the hand-traced ROI contains the whole caudate volume including the insignificantly activated regions, the time course extracted from these box-based ROIs reflected the averaged fMRI hemodynamic response of most significantly activated voxels within the ROI, while the time course extracted from the hand-traced ROI reflected an average of the hemodynamic response of mostly insignificantly activated voxels. Therefore, the averaged BOLD signal was stronger (i.e., the differences of BOLD signal between controls and patients are bigger) in these automated box-based ROIs than that of manual (hand-traced) ROI and such box-based ROIs are more sensitive in detecting group differences than manual ROI in group t-test. The non-uniformity of activation may also explain why the averaged signal amplitudes of fMRI hemodynamic response for all subjects are higher from the automated box ROIs than from the manual ROI. |
| In fMRI ROI analysis, one difficulty is how to measure fMRI signal within the ROI [28]. The program used in this study averages the BOLD responses of the voxels within the ROI and averaging them across all cycles. The practice of averaging responses over voxels across the entire region has the advantages in simplifying analyses and summarizing subject-specific responses without assuming anatomical homology over subjects. On the other hand, it is based on the assumption of functional homogeneity across the ROI and fMRI signals across voxels in the ROI may be functionally heterogeneous (activated or deactivated) [29]. The approach used in this study has weakness in dealing with heterogeneous BOLD responses across the ROI and could cancel out the BOLD responses of voxels with activation and deactivation within the ROI. In addition, it ignores the variations of various time course cycles. Consequently, bias may be introduced into this study. Rather than calculating the mean values, the latest version of SPM extracts the eigenvariate values in a region because eigenvariate values are more robust to heterogeneity of response within the ROI. Another fMRI ROI analysis software FSL-ROI toolbox extracts time courses for each condition at each voxel of the ROI using a finite impulse response (FIR) model. In the SPM and FSL ROI tools, the time courses extracted are not averaged across all trials. Such ROI time course extraction approaches are more advantageous and robust, which needs to be explored in our future studies. |
| Taken together, fMRI studies have revealed apparent functional anomalies in a number of brain disorders [13,30,31]. The possibility of detecting early signs of mental illness with non-invasive fMRI is promising to clinicians and patients. The potential for functional neuroimaging such as fMRI to elucidate brain responses and connectivity may ultimately contribute to clinical practice [32]. Toward that end, methodological exploration and advances in functional neuroimaging (especially fMRI) may release it from unreliability, inaccuracy and inefficiency, and help reach its full potential in clinical settings. |
| Conclusions |
| In summary, we investigated abnormalities of fMRI Hemodynamic Response (HR) in the caudate nucleus in schizophrenia with manual, semi-automated and automated approaches. Compared with controls, less activation (with weak and delayed fMRI HR) in the caudate was observed in the patient group with all of the 3 approaches. High correlations of the AUC were found between the manual and semiautomated ROI approaches, but not between the manual and the automated ROI approach. In addition, the location and size of the automated box ROI are critical for the fMRI hemodynamic response curve extracted, e.g., a larger box-ROI is slightly more highly correlated with the manual tracing. The abnormal fMRI hemodynamic response in the caudate in the patient group may suggest functional deficits in the frontal-striatal-thalamic circuit in schizophrenia. To speed up fMRI data analysis in anatomical ROI, the semi-automated approach may be used as an alternative to the manual approach in detecting fMRI experimental effect. |
| Acknowledgement |
| We are grateful for the cooperation of Department of Radiology at the Mt. Sinai Medical Center. This work is partly supported by NIH grant MH60023, and the Natural Science Foundation of China (Grant No. 81071211). |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14532
- [From(publication date):
November-2013 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9919
- PDF downloads : 4613
