Case Report Open Access
A Large Brunners Gland Hamartoma Caused Gastrointestinal Bleeding: Case Report and Review of Literature
Tugan Tezcaner1*, Yahya Ekici1, Ozgur Harmanci2 and Gokhan Moray3
1Department of General Surgery, Baskent University School of Medicine, Turkey
2Department of Gastroenterology and Hepatology, Baskent University School of Medicine, Turkey
3Department of General Surgery, Baskent University School of Medicine, Turkey
- *Corresponding Author:
- Tugan Tezcaner, MD
Department of General Surgery; Baskent University School of Medicine
5. Sok No: 48 Bahcelievler Ankara, Turkey
Tel: +903122152629
Fax: +9023122234909
E-mail: tugantezcaner@gmail.com
Received date: July 17, 2015 Accepted date: September 01, 2015 Published date: September 10, 2015
Citation: Tezcaner T, Ekici Y, Harmanci O, Moray G (2015) A Large Brunner’s Gland Hamartoma Caused Gastrointestinal Bleeding: Case Report and Review of Literature. J Gastrointest Dig Syst 5:335. doi:10.4172/2161-069X.1000335
Copyright: © 2015 Tezcaner T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Brunner’s gland hamartoma is a rare benign tumor of the duodenum. It may present with either various symptoms caused by obstruction or bleeding; or incidental mass with no symptoms. A 54-years old woman admitted to hospital for melena. Esophagogastroduodenoscopy revealed a large pedunculated polyp arising from distal portion of the duodenal bulb; erosions and hematin coatings was found overlying mucosa. Endoscopic removal was not possible due to its measures and thick stalk. The lesion was resected at the base of stalk with normal mucosa via duodenotomy. Histopathologic examination revealed that this tumor was a Brunner’s gland hamartoma, 5 cm in its greatest dimension. We described a case of Brunner’s gland hamartoma caused upper gastrointestinal bleeding four years after the first diagnosis and related literature was reviewed.
Keywords
Brunner’s gland; Surgical resection; Duodenal polyp
Introduction
Brunner’s glands are located in the deep mucosal or submucosal layers of the most proximal region of the duodenum [1]. Brunner’s gland hamartoma, also known as Brunneroma or polypoid hamartoma, is a rare, benign, proliferative lesion arising from the Brunner’s glands of the duodenum. Benign small bowel tumors are found in 0.16% of cases in autopsy series, and a very small portion of these tumors are Brunner’s gland hamartomas [2]. The natural history of this lesion is not well understood. Gastrointestinal (GI) bleeding is the most common presentation followed by intermittent obstruction, and the lesions can be found incidentally without any symptoms as well [3]. In this study, we reported a Brunner’s gland hamartoma that presented with upper GI bleeding 5 years after initial diagnosis, and presented a short review of literature.
Case Report
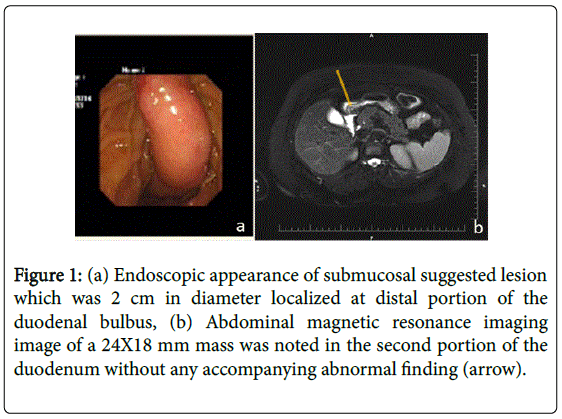
A 50-year-old woman was admitted to hospital with intermittent abdominal pain localized to the epigastrium. An upper GI endoscopy revealed a 2-cm (diameter) submucosal lesion localized at the distal portion of the duodenal bulbus plus antral gastritis (Figure 1a). Biopsies were taken from the overlying mucosa of the lesion, which were non-diagnostic. A magnetic resonance imaging (MRI) scan of the abdomen was performed, and a 24 x 18-mm mass was noted in the second portion of the duodenum without any accompanying abnormal findings. The mass was compatible with lipoma according to MRI findings (Figure 1b). The patient did not want any further investigation or intervention at that time.
Figure 1: (a) Endoscopic appearance of submucosal suggested lesion which was 2 cm in diameter localized at distal portion of the duodenal bulbus, (b) Abdominal magnetic resonance imaging image of a 24X18 mm mass was noted in the second portion of the duodenum without any accompanying abnormal finding (arrow).
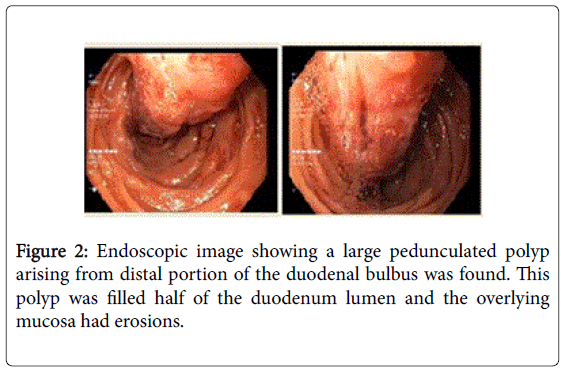
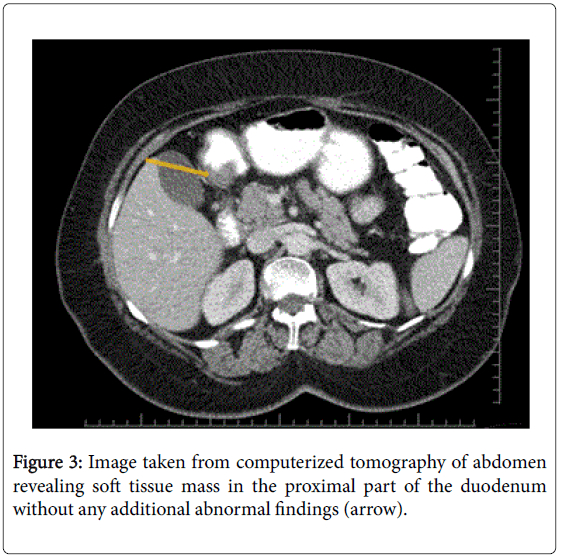
Four years after her first admission, the patient was admitted to the gastroenterology department with upper abdominal pain. Upper GI endoscopy was repeated; a large pedunculated polyp arising from distal portion of the duodenal bulbus was found. This polyp filled half of the duodenal lumen and the overlying mucosa had erosions (Figure 2). Endoscopic removal was not possible due to its size and thick stalk. Multiple biopsies were taken and interpreted as “non-specific duodenitis”. Abdominal computed tomography revealed a soft tissue mass in the proximal part of the duodenum without any additional abnormal findings (Figure 3). The patient was referred to the Department of Surgery. However, she experienced GI bleeding with melena before her surgical appointment could be fulfilled. She was admitted to the emergency department with epigastric fullness and tarry stool. Physical examination revealed no abnormal findings. Blood tests showed low hemoglobin (9.8 g/dL) levels. An emergent upper GI endoscopy was performed because of the patient’s history of duodenal mass, and revealed superficial ulcerations overlying the duodenal mass and hematin coatings suggesting recent bleeding. She was hospitalized for follow-up and showed no signs of bleeding. She agreed to undergo surgery and was taken to the operating room after preoperative preparations. She underwent laparotomy through a right subcostal incision under general anesthesia.
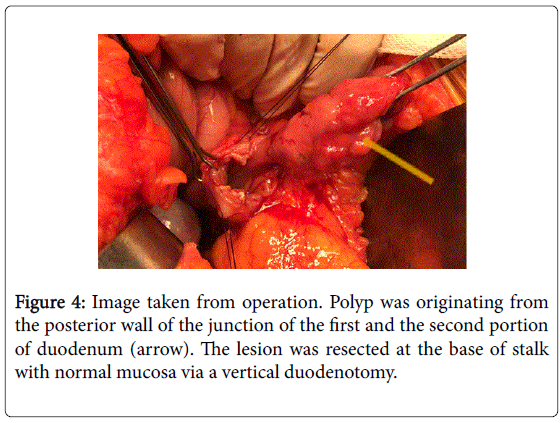
A 5-cm (diameter) mass was found at the junction of the first and second portions of the duodenum. A vertical duodenotomy was performed on the anterior wall of the duodenum where the polyp could be palpated.
The stalk of the polyp originated from the posterior wall of the junction of the first and second portions of the duodenum (Figure 4). The lesion was resected at the base of the stalk with normal mucosa. The frozen sections revealed a possible stromal lesion with no signs of adenocarcinoma. The duodenotomy was closed transversely in order to prevent stricture.
The resected specimen was a lobulated, 5 x 2.5 x 1.5-cm polypoid mass, which contained a lesion measuring 3.5 × 2 × 1.5 cm. The stalk measured 1.5 cm in diameter and 0.5 cm in length.
The tumor was completely enveloped by the intact thin duodenal mucosa. Wide erosions and ulcerations were found on the surface of the tumor. The cut surface of the tumor had a gray-red or gray-yellow color suggesting lobules. The consistency was moderate. Microscopic examination revealed that the tumor was composed of hyperplasia of the Brunner’s glands. The hyperplasia formed lobules that were separated by intervening bands of fibrous tissue, adipose tissue, ducts, and well developed aggregated lymphoid cells. No signs of malignancy were found in the hyperplastic tissue, Brunner’s glands, or surrounding duodenal mucosa. The final pathologic diagnosis was Brunner’s gland adenoma. The patient had an uneventful postoperative course and was discharged on postoperative day 7. She has remained symptom free since intervention, and no episode of recurrent melena has been reported.
Discussion
Brunner’s glands were described for the first time in the late 17th century by a Renaissance pathologist. After his death, this glands were identified as a separate entities from the duodenal glands and termed “Brunner’s glands.” They secrete alkaline mucus as well as enterogastrone to protect the duodenal mucosa from the acid chyme of the stomach.
Since the first Brunner’s gland lesion was reported by Salvioli in 1876, there has been an ongoing debate about the descriptions of the different proliferation types. The proliferation of Brunner’s glands was classified into three categories by Feyrter [4]: (1) diffuse hyperplasia, (2) circumscribed hyperplasia, and (3) glandular adenoma. Robertson [5] explained that these different descriptions reflect manifestations of a common etiology. Brunner’s hamartoma gained its name due to the lack of encapsulation, absence of dysplasia, and mixture of tissues including ducts, acini, smooth muscle, adipose tissue, lymphoid tissue, and sheets of Brunner’s glands associated with the condition.
Reports categorizing Brunner’s gland lesions have been confusing, because the distinctions apply to size rather than histological behavior [6]. Brunner’s gland hyperplasia is an increase in size without the formation of a mass, and leads to the formation of diffuse and focal lesions infiltrating into the wall and circumference of the duodenum. Brunner’s gland hamartoma lesions are made up of nodular proliferation of histologically normal branched acinotubular submucosal mucus-secreting glands and stromal elements [7]. An adenoma of any organ is made up of a glandular structure lined by neoplastic cells, which stand in sharp distinction against the normal tissues. Furthermore, adenomas can show atypia, pleomorphism, mitosis, or even dysplastic changes indicating their neoplastic nature. Brunner’s gland adenomas emerging from neoplasms that undergo malignant transformation are a very common occurrence, since it is natural to assume that the long standing effect of gastric stimuli on these glands will not only lead to severe hyperplasia and dysplasia but also to oncogenesis, especially considering that conditions associated with gastric hyperacidity are common worldwide. However, this is not the case, since duodenal carcinomas are rare and the reported cases are most probably primary duodenal adenocarcinomas or carcinomas arising from the Brunner’s gland rather through malignant transformations of Brunner’s gland adenomas [8,9]. Brunner’s gland hyperplasia nodules with true neoplasm are also rare. Fujimaki et al. [10] demonstrated a focus of p53 positive atypical glands resembling the excretory ducts rather than the acinar cells of the glands, indicating that some lesions may be neoplastic. However, this finding still needs to be substantiated, since the ductal element may be a primary Brunner’s gland lesion rather than secondary to gastric stimuli. An associated microcarcinoid is probably the only report associated with a true neoplasm. Nevertheless, these are incidental findings of associated neoplastic foci unrelated to the pathology of Brunner’s glands.
The cause of Brunner’s gland hamartomas remains unknown. Two theoretical etiologies have been proposed: (1) hyperacidity and (2) pancreatic exocrine insufficiency. Based on the available evidence, hyperacidity itself is unlikely to be the cause, as hyperacidity is not found in all patients with the condition [11] and the hamartomas do not respond to anti-acid therapy [12]. An interesting report evaluated specimens from pancreaticoduodenectomies performed for chronic pancreatitis. The authors found that 76% of patients showed diffuse Brunner’s gland hyperplasia [13]. This may suggest an association between pancreatic exocrine insufficiency and Brunner’s gland hyperplasia.
The clinical presentation of Brunner’s gland hyperplasia may vary from asymptomatic masses incidentally found in the duodenum to obstructive duodenal tumors or bleeding from the mass. Obstructive tumors cause bloating, epigastric fullness, nausea, vomiting, and weight loss. Patients may also have former or initial GI bleeding. Clinicians may also encounter rare presentations such as intussusception or diarrhea.
The diagnosis of a Brunner’s gland hamartoma is challenging due to its non-specific presentation and difficulties in histopathological diagnosis. A differential diagnosis is crucial when a duodenal mass is detected, even if it is found incidentally or based on symptoms. The differential diagnosis of duodenal masses must take into consideration adenomatous polyps, stromal tumors, lymphomas, peri-ampullary carcinomas, duodenal adenocarcinomas, carcinoid tumors, and malign melanomas.
Several diagnostic studies can be performed. In barium X-rays, the findings are often non-specific because there is usually a sessile or pedunculated polypoid-filling defect in the duodenal bulb. Esophagogastroduodenoscopy (EGD) can help with diagnosis but also treatment. EGD is the best method for checking the overlying mucosa. Most Brunner’s gland hamartomas occur in the proximal duodenum; 70% of hamartomas are located in the duodenal bulb [3]. Erosions or ulcers can be noted in this mucosa; multiple mucosal pinch biopsies are mandatory to exclude adenocarcinoma. Unless there are erosions or ulcers, an accurate biopsy must be taken from submucosal tumor tissue. Endosonography (EUS) is superior to other methods for assessing submucosal lesions. Brunner’s gland hamartomas have a number of specific findings that can be detected by EUS: (1) heterogeneous hyperechoic structure, (2) multiple cystic areas, (3) indistinct margins, and (4) originated from the second or third layer [14]. Computed tomography and MRI are not effective for diagnosis alone, but can be helpful for differential diagnosis and delineating the relationship between the hamartoma and the adjacent structures.
A final diagnosis can be determined based on histopathological examination. Histopathological characteristics are non-encapsulated lesions with lobulated Brunner’s glands. Brunner’s glands are combined with ducts, smooth muscle, adipose tissue, sclerotic glands, and lymphoid aggregates [5,15]. Brunner’s gland hamartoma is not considered as a premalignant lesion, although there are reports of focal atypia [16] or microcarcinoid foci within the lesion [17], or transition within the polyp from hyperplastic to adenomatous features [18].
There is no consensus on the management of Brunner’s gland hamartomas. Its benign features and the insufficient knowledge of its natural history make it hard to decide on the need for removal in cases of asymptomatic lesions. However, if the lesions cause complications like obstruction or bleeding, they must certainly be resected with clear margins. Recently, Brunner’s gland hamartomas have been successfully removed endoscopically. The endoscopic approach appears to be less invasive than open surgery, safe, and effective [19]. Limitations of endoscopic treatment are related to the size of the polyp, thickness of the stalk, and localization. The size of the endoscopically resected lesions has ranged from 0.5 cm to >5 cm [20]. Lesions that cannot be removed endoscopically should be referred to surgery. Local surgical resection with clear margins via duodenotomy should be the first choice. Extensive surgical resections are reserved for cases with suspected malignancy or tumors that are too large to allow for endoscopic resection [21,22]. Although long-term follow up data of the removal of Brunner’s gland hamartomas are very limited, there are no reports of recurrence or new tumor development [3].
References
- Zimmermann OC, Brunner JC(2012) Diabetes Its Medical and Cultural History: Outlines—Texts—Bibliography 209.
- Attanoos R, Williams GT (1991) Epithelial and neuroendocrine tumors of the duodenum.SeminDiagnPathol 8: 149-162.
- Levine JA, Burgart LJ, Batts KP, Wang KK (1995) Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases.Am J Gastroenterol 90: 290-294.
- Feyrter F (1934)Überwucherungen der brunnerschendrüsen. VirchowsArchiv 293:509-26.
- Robertson H (1941) The pathology of Brunner's glands. Arch Pathol 31:112-30.
- Al-Hilli FA, Malik AK, George SM (2003) The pathological expressions of Brunners gland hyperplasia into adenoma and hamartoma.Saudi Med J 24: 1256-1260.
- Chong KC, Cheah WK, Lenzi JE, Goh PM (2000) Benign duodenal tumors.Hepatogastroenterology 47: 1298-1300.
- Matsuura H, Kuwano H, Kanematsu T, Sugimachi K, Haraguchi Y (1990) Clinicopathological features of elevated lesions of the duodenal bulb.J SurgOncol 45: 79-84.
- Akino K, Kondo Y, Ueno A, Yamazaki K, Hosokawa M, et al. (2002) Carcinoma of duodenum arising from Brunner's gland.J Gastroenterol 37: 293-296.
- Fujimaki E, Nakamura S, Sugai T, Takeda Y (2000) Brunner's gland adenoma with a focus of p53-positive atypical glands.J Gastroenterol 35: 155-158.
- Kaplan E, Dyson W, Fitts W (1968) Hyperplasia of brunners glands of duodenum. Surgery gynecology and obstetrics with international abstracts of surgery franklin h martin foundation 55 e eriest, Chicago 60611: 371-373.
- Spellberg MA, Vucelic B (1980) A case of Brunner's glands hyperplasia with diarrhea responsive to cimetidine.Am J Gastroenterol 73: 519-522.
- Stolte M, Schwabe H, Prestele H (1981) Relationship between diseases of the pancreas and hyperplasia of Brunner's glands.Virchows Arch A PatholAnatHistol 394: 75-87.
- Changchien CS, Hsu CC, Hu TH (2001) Endosonographic appearances of Brunner's gland hamartomas.J Clin Ultrasound 29: 243-246.
- Akaki M, Taniguchi S, Hatakeyama K, Kushima R, Kataoka H (2014) Duodenal mucosal damage is associated with proliferative activity of Brunner's gland hamartoma: a case report.BMC Gastroenterol 14: 14.
- Fujimaki E, Nakamura S, Sugai T, Takeda Y (2000) Brunner's gland adenoma with a focus of p53-positive atypical glands.J Gastroenterol 35: 155-158.
- Matsui T, Iida M, Fujischima M, Sakamoto K, Watanabe H (1989) Brunner's gland hamartoma associated with microcarcinoids.Endoscopy 21: 37-38.
- Suda K, Hamada S, Takahashi M, Kumashiro Y, Yao T, et al. (2007) A true Brunner's gland adenoma.Endoscopy 39 Suppl 1: E37-38.
- Walden DT, Marcon NE (1998) Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma: case report and expanded literature review.GastrointestEndosc 47: 403-407.
- Huang CH, Perng CL, Yang YH, Shyr YM, Lin HJ, et al. (1999) Endoscopic removal of a huge duodenal Brunner's gland adenoma: a new technique.GastrointestEndosc 50: 868-869.
- Iusco D, Roncoroni L, Violi V, Donadei E, Sarli L (2005) Brunner's gland hamartoma: 'over-treatment' of a voluminous mass simulating a malignancy of the pancreatic-duodenal area.JOP 6: 348-353.
- Sen R, Gupta V, Sharma N, Chawla N, Kumar S, et al. (2014) Brunner gland hamartoma masquerading as malignancy; a rare case report.Middle East J Dig Dis 6: 237-240.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14751
- [From(publication date):
October-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10226
- PDF downloads : 4525