A Hot-Water Extract of Spirulina platensis Enhances Spontaneous Cytotoxic T cell Activation in Mouse Tumor Models
Received: 11-Aug-2020 / Accepted Date: 24-Aug-2020 / Published Date: 31-Aug-2020
Abstract
Spirulina platensis is a cyanobacteria species that contains a TLR2 agonist and phycocyanin that supports probiotic health care through innate immunity. The oral administration of hot water-extracted spirulina (spirulina extract) induced NK cell activation and shrunk the NK-sensitive tumors loaded onto mice. However, it remains undetermined whether spirulina extract contributes to provoking T cell immunity represented by the tumor-specific induction of cytotoxic T lymphocytes (CTLs). Using a mouse model comprised of mice with CTL-sensitive tumors and a diet consisting of spirulina extract, we found that tumor-specific CTLs were spontaneously activated by spirulina extract in some mice, in which tumor regression was observed in a CTL-dependent manner. CTLs were induced without an injection of extrinsic tumor-associated antigen (TAA). These results indicated that the spirulina extract affected the activation of both NK cells and CTLs in the gut microbial environment to induce tumor regression in some mice when the tumor provided sufficient TAA.
Keywords: Spirulina; Tumor regression; CTL
Introduction
The immune system has innate and acquired arms of the immune response to discriminate non-self from self cells. The innate system recognizes pattern recognition molecules as non-self signature markers: pattern-recognition receptors (PRRs) expressed on host cells. PRRs are distributed throughout the cells of the body and signal the presence of foreign invaders by producing cytokines and inflammatory mediators. This acute phase response helps immune cells eliminate pathogenic microbes which possess pathogen-associated molecular patterns (PAMPs) [1]. In vertebrates, lymphocytes further endow the immune system with specificity and memory. Notably, innate sensing precedes the activation of the acquired immune response.
Although innate sensing provides a series of mediators to sufficiently alarm the presence of microbes, precise recognition for the elimination of microbes is accomplished by antigen-specificity associated with the acquired immune system, which consists of T and B lymphocytes. Moreover, these lymphocytes display clonal diversity to cover the huge variation of possible foreign antigens. The clonal expansion of T and B cells is an essential event to facilitate the specific elimination of microbes. The most critical cell that can trigger clonal T cell proliferation is dendritic cells (DCs) [2]. PRR expression in DCs triggers the activation of transcription factor signaling (e.g., AP- 1, NF-κB, and IRFs) in these cells [3]. Several cytokines and cellular effectors are consequently induced to orchestrate the host defense. DCs sufficiently activate T cells: cis-activation by DC cytokines and inflammatory mediators, which have been designated for DC priming [2]. In addition, MHC and co-stimulatory factors (e.g., CD80 and CD86) are upregulated in DCs, which are markers for DC-priming. Moreover, DC-priming provides the basis for T cell proliferation.
We have a variety of foods that may contain several types of microbial patterns. Because intestinal and colon epithelial cells express PRRs [4,5], mucosal immune responses may be modulated by the oral uptake of microbial material; however, most dietary molecules do not induce a systemic immune response. Although the reason for this effect remains unknown, it is presumably due in part to the complexity of the gut-associated lymphoid tissue (GALT), which typically provides immune tolerance to dietary antigens of food origin [6].
The cyanobacterium, Spirulina platensis (spirulina), has been ingested as a supplemental food for more than 40 years without any undesirable side-effects [7]. Spirulina is considered safe for human consumption, which has been confirmed through toxicological studies. Spirulina contains a lipopolysaccharide (LPS)-like constituent that is structurally different from bacterial LPS [8]. In addition, spirulina also contains a high protein content, including phycocyanin, vitamins (i.e., A and B12), and minerals. It is also rich in phenolic acids, tocopherols, and γ-linolenic acid [7,9]. Since spirulina lacks a cell wall, it is easily digested [7,9]. Spirulina contains unique proteins, sugars, and lipids [7,10], and these moieties have been reported to participate in inducing the host immune response, including enhancing Ab production [11], cytokine liberation [12], T-cell responses, and NK activation [13]. However, the precise molecular mechanism responsible for this immune response has not been clearly identified to date.
We previously reported that the hot-water extract of spirulina enhanced NK activation when taken orally in elderly volunteers [13]. The activity of spirulina is unique because most of the reported bacterial adjuvants facilitate CTL induction but not NK activation [14]. The majority of immune-enhancing adjuvants are subcutaneously injected to induce acute inflammation and IL-12 production. DC-priming is followed by the activation of cellular immunity, which induces increased production of IFN-γ, a marker of lymphocyte activation. DC-mediated NK activation is another marker of DC-priming [15,16]. TLRs and the may be an event triggered by DC-priming [2,17]. It is known that most inflammatory adjuvants drive DCs to elicit a CTL-activated state [17].
Here, we show that the hot-water extract of spirulina orally administered activates not only NK cells but also CD8 T+cells in >30% of mice bearing ovalbumin (OVA)-expressing tumor. Consequently, spirulina diet led to the suppression of implant tumor growth in the mice. Thus, spirulina evokes unique MyD88-dependent DC-priming through the mucosal immune response, at least when antigens are intrinsically provided.
Materials and Methods
Reagents
Spirulina extract was provided by DIC Corporation (Tokyo, Japan) and prepared as previously described [13] with a slight modification. 200 μl of spirulina extract (corresponding to 96 mg of spirulina powder) was administrated to mice at a time. Low Endotoxin ovalbumin (OVA) was purchased from Fuji Film-Wako (Tokyo, Japan). Dulbecco’s modified PBS (D-PBS) used to substrate solution or injection into mice was endotoxin-free grade (MER Millipore, Darmstadt, Germany). 2,3-bis (palmitoyl) propyl Cys-Ser-Lys-Lys-Lys-Lys (Pam2CSK4) was synthesized by Biologica Co. Ltd (Nagoya, Japan). Anti-PD-L1 antibody (Ab) (clone: 10F.9G2) and rat IgG2b isotype control Ab (LTF- 2) were purchased from Bio × Cell (West Lebanon NH, USA). 7-amino actinomycin D (7AAD, Cell : bility solution) for dead cell staining was purchased from BD Biosciences (Franklin Lakes, NU, USA). Abs used for flow cytometry are listed in (Table 1).
| Antibody | Clone | Supplier, Catalog number |
|---|---|---|
| Anti-mouse CD16/32 | 93 | Biolegend, 101302 |
| FITC-anti-CD45 | 30F11 | Biolegend, 103108 |
| FITC-anti-CD4 | GK1.5 | Biolegend, 100406 |
| FITC-anti-mouse CD3 | 145-2C11 | Biolegend, 100306 |
| FITC-anti-mouse F4/80 | BM8 | Biolegend, 123107 |
| FITC-anti-Ly6C | HK1.4 | Biolegend, 128005 |
| FITC-anti-mouse CD86 | GL-1 | Biolegend, 105005 |
| FITC-anti-mouse H-2Kb/H-2Db | 28-8-6 | Biolegend, 114605 |
| PE-anti-mouse CD3ε | 145-2C11 | Biolegend, 100308 |
| PE-anti-CD69 | H1.2F3 | Biolegend, 100311 |
| PE-anti-Gr-1 | RB6-8C5 | Biolegend, 123107 |
| PE-anti-CD103 | 2.00E+07 | Biolegend, 121405 |
| PE-anti-mouse CD80 | 16-10A1 | Biolegend, 104707 |
| PE-anti-mouse CD40 | 1C10 | eBioscience, 12-0401-81 |
| PE-anti-mouse H-2Kb/H-2Db | 28-8-6 | Biolegend, 114607 |
| PE-anti-mouse TCRVβ5.1/5.2 | MR9-4 | Biolegend, 139503 |
| PE-anti-mouse PD-L1 | M1H5 | eBioscience, 12-5982-81 |
| PE/Cy7-anti-mouse CD3 | 17A2 | Biolegend, 100219 |
| PE/Cy7-anti-CD11b | M1/70 | Biolegend, 101215 |
| PE/Cy7-anti-CD11c | N418 | Biolegend, 117318 |
| APC/Cy7-CD45 | 30-F11 | Biolegend, 103116 |
| APC-CD45.2 | 104 | Biolegend, 109814 |
| APC-anti-mouse CD3ε | 145-2C11 | Biolgend, 100311 |
| APC-anti-mouse CD8α | 53-6.7 | Biolegend, 100712 |
| APC-anti-NK1.1 | PK136 | eBioscience, 17-5941-82 |
| APC-anti-F4/80 | BM8 | Biolegend, 123115 |
| APC-anti-Gr-1 | RB6-8C5 | Biolegend, 108411 |
| AF700-anti-mouse CD3 | 17A2 | Biolegend, 100216 |
| AF700-anti-mouse CD8α | 53-6.7 | Biolegend, 100730 |
| FITC-Rat IgG2aκ | RTK2758 | Biolegend, 400505 |
| PE-Hamster IgG | HTK888 | Biolegend, 400907 |
| FITC-Mouset IgG2aκ | eBM2a | eBioscience, 11-4724-41 |
| PE-Rat IgG2aκ | RTK2758 | Biolegend, 400507 |
Table 1: List of antibodies for flow cytometry.
Mice
Inbred female wild-type C57BL/6 mice were purchased from Clea Japan. Mice were maintained under specific pathogen-free conditions and used in the age 7-11 weeks. OT-I mice were kindly provided by Dr. N. Ishii (Tohoku University, Sendai, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee of Hokkaido University (the number was 18-0032) and performed in compliance with their guidelines.
Cells
OVA-expressing B16 melanoma cells (MO5) was kindly provided by Dr. H. Udono (Okayama University, Okayama, Japan) [18] and cultured in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% heatinactivated fetal bovine serum (FBS, purchased from GE Healthcare Life Sciences, Buckinghamshire, England), 100 IU of penicillin/100 μg/ ml of streptomycin (Thermo Fisher Scientific, Waltham, MA, USA), and 100 μg/ml of G418 (Roche). OVA-expressing EL4 lymphoma cells (EG7) (ATCC®CRL-2113TM) were purchased from ATCC (Manassas, VA, USA) and cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS, 10 mM of HEPES (Thermo Fisher Scientific), 1 mM of sodium pyruvate (Thermo Fisher Scientific), 55 μM of 2-mercaptoethanol (Thermo Fisher Scientific), 100 IU of penicillin/100 μg/ml of streptomycin and 500 μg/mL of G418.
OT-I proliferation assay
Splenocytes were isolated from spleens of two mice administrated with H2O or spirulina extract by treatment with collagenase D (Roche Diagnostics, Mannheim, Germany). CD11c+ DC cells were isolated from the splenocytes by CD11c-microbeads (MiltenyiBiotec, Auburn, CA, USA) under blocking with anti-CD16/CD32 Abs. OT-I T cells were isolated from spleens of OT-I mice by CD8-microbeads (MiltenyiBiotec.). The isolated cells were labeled with 1 μM of carboxyfluoresceindiacetatesuccinimidyl ester (CFSE, Thermo Fisher Scientific) for 10 min at 37°C. 1 × 105 cells of CD11c+ cells were seeded in a well of U-bottom 96-well plate in triplicate, and incubated with or without 2.5 μg/ml of OVA at 37°C. After 4 hr, equal volume of CFSElabeled OT-I T cells containing 5 × 104 cells were added into each well, and cells were cocultured for 62 hr at 37°C. The cells were stained with anti-CD8α, anti-CD3 and anti-TCR Vβ5.1, 5.2 Abs. Dead cells were excluded by 7AAD staining. OT-I T cells proliferation was evaluated by the attenuation of CFSE by FACS AriaII (BD Biosciences) and Flowjo software (Tree Star, CA, USA).
Analysis of tumor microenvironment
For analysis of intratumor immune cells, tumor tissues were finely minced and treated with 0.05 mg/ml collagenase I (Sigma-Aldrich, ST. Louis, MI, USA), 0.05 mg/ml collagenase IV (Sigma-Aldrich), 0.025 mg/ml hyaluronidase (Sigma-Aldrich), and 0.01 mg/mlDNase I (Roche Diagnostics) in Hank’s Balanced Salt Solution (Sigma-Aldrich) at 25°C for 15 min. After enzyme treatment, red blood cells were lysed with ACK buffer. Tumor-infiltrating CD8+ T cells, CD4+ T cells and NK cells were stained with antibodies described in Table 1 and analyzed by FACS AriaII and Flowjo software.
Tumor challenge and spirulina administration
MO5 cells or EG7 cells (1 × 106 cells or 2 × 106 cells/200 μl D-PBS) were subcutaneously (s.c.) injected into shaved back of mice. Tumor size was measured using calipers and determined by following formula: tumor volume (mm3)=0.52 × (long diameter) × (short diameter)2.
Orally administration of 200 μl of spirulina extract or H2O by sonde was started when the tumor volume reached around 200 mm3. The administration was performed every two or three days. When the animal experiments were performed with different protocol from above, we described the details in the figure legends. For the CD8β cells depletion, 15 μl of hybridoma ascites containing anti-CD8β monoclonal Ab (clone: H35.17-2) was intraperitoneally (i.p.) injected into mice 1 day before spirulina administration. Ascites generally contains 10-15 mg/ml of IgG [19]. 200 μg of isotype control Ab or anti-PD-L1 Ab was i.p. injected into spirulina-administrated mice on day 6, 8 and 10 after tumor challenge. Mice was euthanized when a tumor volume reached to 3,000 mm3.
Results
Tumor growth suppression induced by spirulina extract in melanoma line MO5
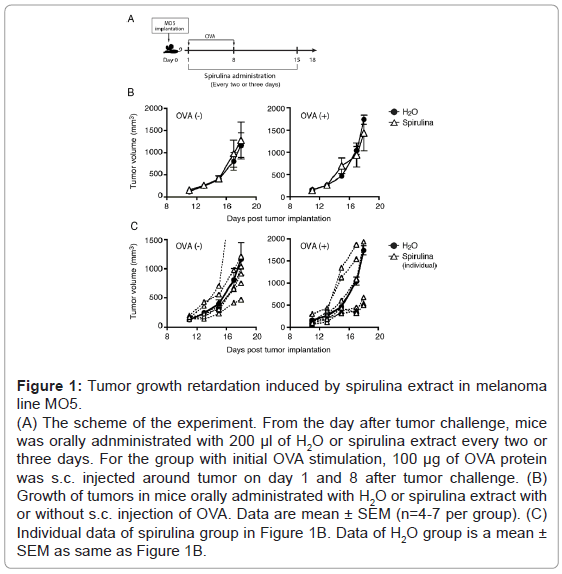
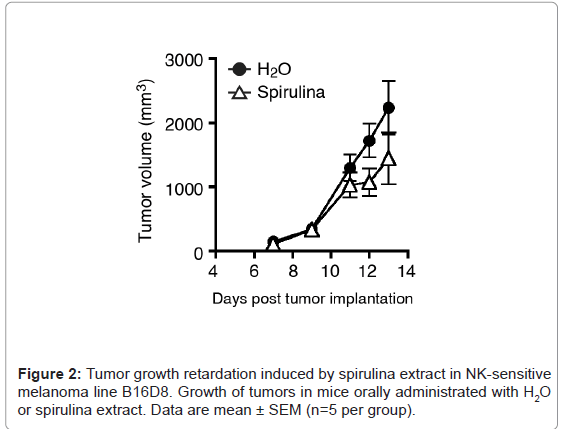
Several sublines with a putative tumor antigen, OVA, were established in B16 melanoma cells, which is syngeneic with C57BL/6 mice. Both MO5 and B16D8 lines are B16 sublines, and MO5 is OVApositive. We subcutaneously (s.c.) injected MO5 cells into shaved back of the mice (Figure 1A). On average, the MO5 tumors grew similarly in the subcutaneous region in both the spirulina and control groups (Figure 1B). NK-mediated tumor regression was observed following treatment with spirulina extract in the B16D8 melanoma subline (Figure 2), which have been reported as NK-sensitive [20].Yet, tumor regression was barely observed in the MO5 melanoma line in response to spirulina extract (Figure 1B). However, in individual cases receiving spirulina extract, some mice showed tumor regression while others actually enhanced tumor progression: there was individual-to-individual difference in spirulina response in this mouse assay. This tendency was more clearly observed in mice with OVA administration (Figure 1C). It was previously reported [20] that B16D8 tumors uniformly undergo growth suppression following treatment with spirulina extract, which reflects NK cell activation. Thus, we speculate that factor (s) other than NK cells participate in MO5 tumor suppression in some individual mice. In reports of MO5 tumors, the factor that is most likely to suppress tumor growth is the CTL response [21].
Figure 1: Tumor growth retardation induced by spirulina extract in melanoma line MO5. (A) The scheme of the experiment. From the day after tumor challenge, mice was orally adnministrated with 200 μl of H2O or spirulina extract every two or three days. For the group with initial OVA stimulation, 100 μg of OVA protein was s.c. injected around tumor on day 1 and 8 after tumor challenge. (B) Growth of tumors in mice orally administrated with H2O or spirulina extract with or without s.c. injection of OVA. Data are mean ± SEM (n=4-7 per group). (C) Individual data of spirulina group in Figure 1B. Data of H2O group is a mean ± SEM as same as Figure 1B.
Suppression of tumor growth is induced by spirulina extract in CTL-sensitive EG7 cells
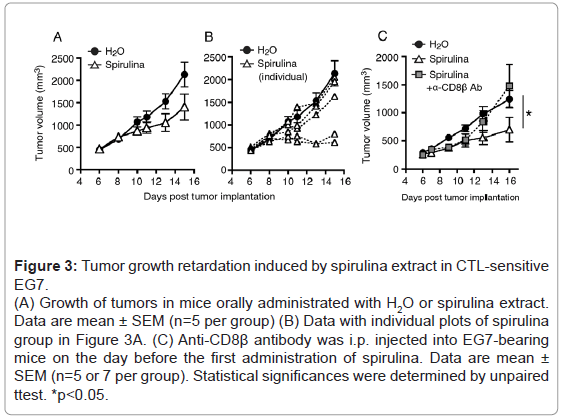
To consolidate the tumor regression induced by spirulina extract, we decided to employ the EG7 lymphoma cell line. EG7 cells express OVA as an antigen, and are susceptible to vaccine immunotherapy with OVA+polyI: C [22]. EG7-loaded mice were challenged with spirulina extract, and tumor growth retardation was observed in several mice in the spirulina-treated group, although there was no significant difference between the control and treated groups (Figure 3A).Only two individual mice showed a prominent decrease in tumor size in the spirulina-treated group (Figure 3B).
Figure 3: Tumor growth retardation induced by spirulina extract in CTL-sensitive EG7. (A) Growth of tumors in mice orally administrated with H2O or spirulina extract. Data are mean ± SEM (n=5 per group) (B) Data with individual plots of spirulina group in Figure 3A. (C) Anti-CD8β antibody was i.p. injected into EG7-bearing mice on the day before the first administration of spirulina. Data are mean ± SEM (n=5 or 7 per group). Statistical significances were determined by unpaired ttest. *p<0.05.
In this context, we tested whether the CTL response was involved in tumor growth suppression. EG7-loaded mice were pretreated with an anti-CD8β Ab, and the mice were treated with spirulina extract (Figure 3C). In the spirulina-treated group, tumor growth was delayed in response to spirulina, and CD8 depletion resulted in the recovery of tumor growth in the spirulina+anti-CD8β Ab group (Figure 3C). Thus, CTLs were considered to be involved in tumor growth suppression in this system.
Mechanism of CTL proliferation by spirulina
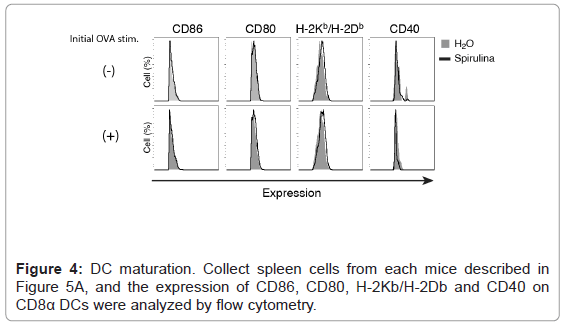
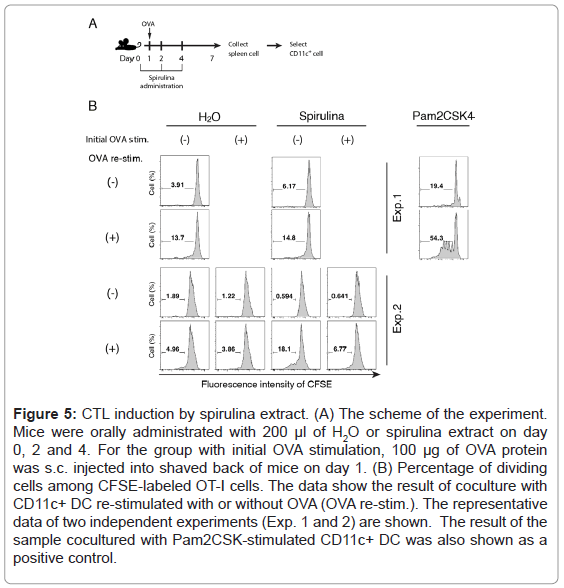
We then investigated the mechanism by which the oral administration of spirulina induced T cell proliferation in an EG7 (OVA) model. If spirulina displays dendritic cell (DC) adjuvant activity in a mucosal environment, DCs move from the GALT to the lymph nodes or spleen to proliferate or activate CD8+ T cells. However, because we could not detect the up-regulation of DC maturation markers, CD80/ CD40/CD86 or H-2Kb/H-2Db, in splenic DCs, this was not the case (Figure 4). Nevertheless, an OVA-specific CTL response was induced by splenic DCs from some spirulina-treated mice (Figure 5). The results were confirmed with an in vitro assay using a mixture of the splenic CD11c and OT-I CD8+ T cells. In the experiment 2 (Figure 5B), DCs from spirulina-treated mouse induced CD8+ T cell proliferation after re-stimulation with OVA, which was occurred irrespective of an initial OVA stimulation. These results indicated that spirulina may up-regulate the antigen cross-presentation by DC without beforehand extrinsic stimulation by antigen.
Figure 5: CTL induction by spirulina extract. (A) The scheme of the experiment. Mice were orally administrated with 200 μl of H2O or spirulina extract on day 0, 2 and 4. For the group with initial OVA stimulation, 100 μg of OVA protein was s.c. injected into shaved back of mice on day 1. (B) Percentage of dividing cells among CFSE-labeled OT-I cells. The data show the result of coculture with CD11c+ DC re-stimulated with or without OVA (OVA re-stim.). The representative data of two independent experiments (Exp. 1 and 2) are shown. The result of the sample cocultured with Pam2CSK-stimulated CD11c+ DC was also shown as a positive control.
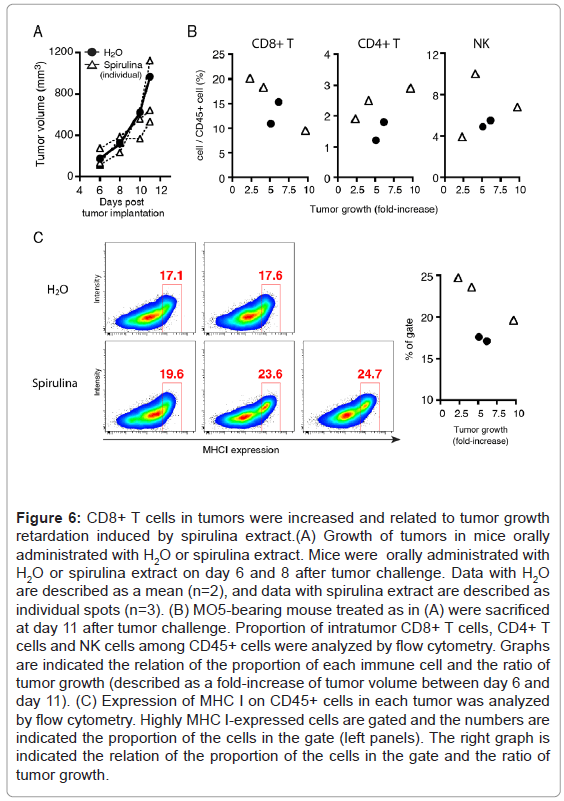
CTL proliferation was also involved in the tumor regression(Figures 6A and 6B) in MO5-bearing mice. CD8+ T cells but not CD4+ T cells or NK cells, were increased in the tumors of spirulina-treated mice with the suppression of tumor growth, and the ratio of CD8+ T cells in the tumor was substantially increased concomitant with tumor regression. Moreover, CD45+ cells with high MHC I expression were found to have infiltrated into the tumors of the spirulina-treated mice, and furthermore the proportion of those cells was reciprocally correlated with tumor growth (Figures 6C).These results indicated the possibility for the induction of cross-presentation of DC and subsequent CTLproliferation in spirulina-treated mice.
Figure 6: CD8+ T cells in tumors were increased and related to tumor growth retardation induced by spirulina extract.(A) Growth of tumors in mice orally administrated with H2O or spirulina extract. Mice were orally administrated with H2O or spirulina extract on day 6 and 8 after tumor challenge. Data with H2O are described as a mean (n=2), and data with spirulina extract are described as individual spots (n=3). (B) MO5-bearing mouse treated as in (A) were sacrificed at day 11 after tumor challenge. Proportion of intratumor CD8+ T cells, CD4+ T cells and NK cells among CD45+ cells were analyzed by flow cytometry. Graphs are indicated the relation of the proportion of each immune cell and the ratio of tumor growth (described as a fold-increase of tumor volume between day 6 and day 11). (C) Expression of MHC I on CD45+ cells in each tumor was analyzed by flow cytometry. Highly MHC I-expressed cells are gated and the numbers are indicated the proportion of the cells in the gate (left panels). The right graph is indicated the relation of the proportion of the cells in the gate and the ratio of tumor growth.
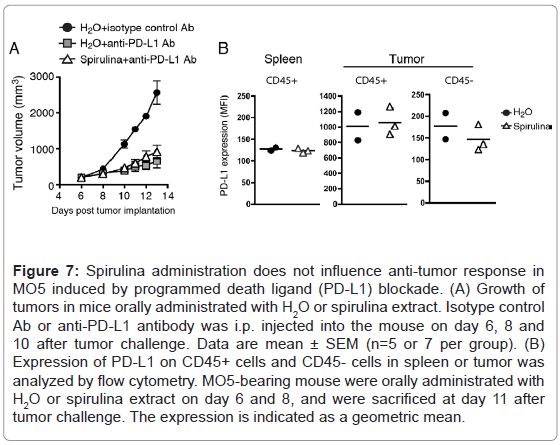
However, PD-L1 blockade therapy was barely enhanced by the combined administration of spirulina in MO5-bearing mice (Figure 7A). In this case, intrinsic antigen-presentation by DCs may not involve PD-L1-mediated regulation of T cell proliferation. The PD-L1 levels on tumor and spleen cells were not affected in response to spirulina diet (Figures 7B).
Figure 7: Spirulina administration does not influence anti-tumor response in MO5 induced by programmed death ligand (PD-L1) blockade. (A) Growth of tumors in mice orally administrated with H2O or spirulina extract. Isotype control Ab or anti-PD-L1 antibody was i.p. injected into the mouse on day 6, 8 and 10 after tumor challenge. Data are mean ± SEM (n=5 or 7 per group). (B) Expression of PD-L1 on CD45+ cells and CD45- cells in spleen or tumor was analyzed by flow cytometry. MO5-bearing mouse were orally administrated with H2O or spirulina extract on day 6 and 8, and were sacrificed at day 11 after tumor challenge. The expression is indicated as a geometric mean.
Discussion
In the present study, we demonstrated that some mouse populations induced growth suppression of OVA-positive tumor in concert with CD8+ CTL proliferation. Previous reports indicated that NK cells were effectors for tumor damage and spirulina is an NK cell activator [20]. There are DC-dependent and independent mediators of NK cell activation [16]. NK-mediated killing depends on the level of tumor Rae-1 [23], which is recognized by NKG2D expression on myeloid cells, including DCs, and DC-dependent NK activation occurs in mice fed a spirulina diet with an NK-sensitive tumor [20]. This finding suggests that DC-priming precedes NK cell activation in the context of a spirulina diet.
If DC-priming is the case for spirulina diet, it is no wonder that spirulina-mediated DC-priming involves the DC-mediated proliferative response of T cells towards antigens [2]. DCs are classified into multiple different subsets, some of which are distributed in the GALT. CD103+ DCs and CD8α DCs are typical antigen-presenting cells (APCs) in mice and are localized in the lymph nodes of the GALTs. It is likely that spirulina-stimulated APCs gain access to GALT-associated lymphocytes. When a tumorharbors TAAs, DC-priming induces the most crucial function of DCs, namely the cross-presentation of extrinsic tumor antigens, which enables DCs to present foreign antigens to CD8+ T lymphocytes, CTLs. Notably, sufficient antigen densities are at least needed to allow APCs to cross-present ectopic antigens that exist distantly from APCs. The current consensus is that no CTLs proliferate without providing antigen peptides and adjuvants in immunotherapy. In spirulina diet, DC-priming and OVA-specific CTL happens only in a minor population under unpredictable conditions. According to the concept of cancer immunotherapy, the data can be interpreted to indicate that dietary spirulina extract works as an adjuvant for tumor-releasing OVA to facilitate CTL proliferation occasionally. The spatiotemporal variations of OVA antigen levels released from tumor would be crucial for each individual mouse. In this study, we could not verify this issue in each mouse.
On the other hand, several reports have described spontaneous tumor shrinkage without the administration of antigens [24,25]. In mouse models, spontaneous tumor regression occurs in CTL-sensitive tumors. In this case, the depletion of CD8β T cells resulted in tumor progression, suggesting the importance of CTLs to shrink tumors. In addition, anti-PD-1 Ab immunotherapy is thought to be effective only in patients with already excited TAA-induced CTLs [26,27]. Moreover, there have been rare reported cases of spontaneous diminution of CTLsensitive implant tumors in mice [28] and cancer in human patients [24,25]. The latter cases typically occur following infection, radiation, or chemotherapy. While it is rare to encounter cases of abscopal effect, such cases surely exist. Thus, microbial pattern molecules may have been enriched in the tumorstroma to sufficiently activate myeloid cells, which leads to the proliferation of TAA-specific CTLs.
The scenario reminds us of the fact that TAA-specific CTLs spontaneously occurring in patients with cancer; however, CTL function is suppressed in the tumor environment [29]. It is likely that TAAs leak out of the tumor to spontaneously activate CTLs in approximately 20% of patients with progressive cancer, which are termed a PD-1 antibody-sensitive tumor. It has been speculated that spirulina has an LPS-like product and the spirulina extract contains a TLR2/4 ligand [20]. However, the PD-L1 levels were not increased in spleen and tumor in spirulina-diet mice in contrast to TLR2-stimulated mice [30-32]. Immune effectors damage tumors, and dying tumor cells discharge TAAs, which results in DC-mediated CTL activation. Notably, CTL proliferation occurs without the injection of TAA peptides in those cases. The type of adjuvant or endogenous substance that can induce DC-priming without external addition of TAA is a fascinating question that requires further investigation.
Conclusion
We could not verify prediction markers for tumor regression in spirulina-diet mice, since tumor regression occurs rare and unpredictable. According to the mouse models, the immunoediting theory suggests that the spirulina diet breaks a stable “Equilibrium” to make a shift towards ‘Elimination’. Both tumor cells and immune cells participate in the formation of tumor environment. Immuneenhancement appears to be associated with abscopal as well as spirulina effect in human intervened studies. In this context, some type of adjuvants may function as NK-stimulants, as well as inducers of a TAA-specific CTL response. Several issues including multiple regulatory factors in DC cross-presentation and the mechanism by which endogenous antigens are internalized and processed in APCs need to be addressed in the future.
Acknowledgement
We appreciate the support of Dr. M. Fukushima (Translational Research Center for Medical Innovation (TRI), Kobe) for their administration support. We thank Mrs. H. Nishimura and A. Yamanaka (TRI, Kobe) for their management support. We also thank Yushinkai Medical Corporation for their general support. This work was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Number JP19ck0106480. This work was in part supported by the Smoking Research Foundation. We had a non-profit financial support from Advanced Innovation Development (AID) Co., Ltd (Tokyo, Japan) and DIC Co. Ltd (Tokyo, Japan) through the university-company contract, which we acknowledge gratefully.
Conflicts of Interest
The authors declare that there is no conflict of interest
References
- Medzhitov R, JanewayCA Jr (1997) Innate immunity: The virtues of a nonclonal system of recognition. Cell 91: 295-298.
- Eisenbarth SC (2019) Dendritic cell subsets in T cell programming: Location dictates function. Nat Rev Immunol 19: 89-103.
- Takeda Y, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21: 335-376.
- McClure R, Massari P (2014) TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol 5: 386.
- Lei-Leston AC, Murphy AG, Maloy KJ (2017) Epithelial Cell Inflammasomes in Intestinal Immunity and Inflammation. Front Immunol 8: 1168.
- Faria AM, Gomes-Santos AC, Gonçalves JL, Moreira TG, Medeiros SR, et al. (2013) Food components and the immune system: From tonic agents to allergens. Front Immunol 4: 102.
- Belay A (2007) Spirulina (Arthrospira): Production and quality assurance. In: Spirulina in human nutrition and health. Gershwin ME, Belay A (eds)Â CRC Press, London, UK. Pp: 1-25.
- Tornabene TG, Bourne TF, Raziuddin S, Ben-Amotz A (1985) Lipid and lipopolysaccharide constituents of cyanobacterium Spirulina platensis (Cyanophyceae, Nostocales). Marine Ecology 22: 121-125.
- Wu Q, Liu L, Miron A, KlÃmová B, Wan D, et al. (2016) The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol 90: 1817-1840.
- Dillon JC, Phuc AC, Dubacq JP (1995) Nutritional value of the alga Spirulina. World Rev Nutr Diet 77: 32-46.
- Hayashi O, Katoh T, Okuwaki Y (1994) Enhancement of antibody production in mice by dietary Spirulina platensis. J NutrSciVitaminol 40: 431-441.
- Mao TK, Van de Water J, Gershwin ME (2005) Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food 8: 27-30.
- Hirahashi T, Matsumoto M, Hazeki K, Saeki Y, Ui M, et al. (2002) Activation of the human innate immune system by spirulina: Augmentation of interferon gamma production and NK cytotoxicity by oral administration of spirulina. IntImmunopharmac 2: 423-434.
- Seya T, Akazawa T, Matsumoto M, Begum NA, Azuma I, et al. (2003) Innate immune therapy for cancer: Application of BCG-CWS and spirulina to patients with lung cancer. Anticancer Res 23: 4369-4376.
- Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K.
- Azuma M, Sawahata R, Akao Y, Ebihara T, Yamazaki S, et al. (2010) The peptide sequence of diacyllipopeptides determines dendritic cell TLR2-mediated NK activation. PLoS One. 5: e12550.
- Akazawa T, Masuda H, Saeki Y, Matsumoto M, Takeda K, et al. (2004) Adjuvant-mediated tumor regression and tumor-specific CTL induction are impaired in MyD88-deficient mice. Cancer Res 64: 757-764.
- Ryu MS, Woo MY, Kwon D, Hong AE, Song KY, et al. (2014) Accumulation of cytolytic CD8+T cells in B16-melanoma and proliferation of mature T cells in TIS21-knockout mice after T cell receptor stimulation. Exp Cell Res 327: 209-221.
- Noeman SA, Misra DN, Yankes RJ, Kunz HW, Gill Tj (1982) Growth of Rat-mouse hybridomas in nude mice and nude rats. J Immunol Methods 55: 319-326.
- Akao Y, Ebihara T, Masuda H, Saeki Y, Akazawa T, et al. (2009) Enhancement of antitumor natural killer cell activation by orally administered Spirulina extract in mice. Cancer Sci 100: 1494-1501.
- Takeda Y, Kataoka K, Yamagishi J, Ogawa S, Seya T, et al. (2017) A TLR3-Specific Adjuvant Relieves Innate Resistance to PD-L1Blockade without Cytokine Toxicity in Tumor Vaccine Immunotherapy. Cell Rep 19: 1874-1887.
- Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T (2012) Cross-priming for antitumor CTL induced by soluble Ag+polyI: C depends on the TICAM-1 pathway in mouse CD11c(+)/CD8α(+) dendritic cells. Oncoimmunology 1: 581-592.
- Masuda H, Saeki Y, Nomura M, Shida K, Matsumoto M, et al. (2002) High levels of RAE-1 isoforms on mouse tumor cell lines assessed by anti-"pan" RAE-1 antibody confer tumor susceptibility to NK cells. BiochemBiophys Res Commun 290: 140-145.
- Kodama K, Higashiyama M, Okami J, Tokunaga T, Fujiwara A, et al. (2014) A possible abscopal effect of post-radiation immunotherapy in two patients with metastatic lung tumors. Int Cancer Conf J 3: 122-127.
- Park SS, Dong H, Liu X, Harrington SM, Krco CJ, et al. (2015) PD-1 restraints radiotherapy-induced abscopal effect. Cancer Immunol Res 3: 610-619.
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, et al. (2012) Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 18: 6497-6508.
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, et al. (2017) An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547: 217-221.
- Zito G, Saotome I, Liu Z, Ferro EG, Sun TY, et al. (2014) Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat Commun 5: 3543.
- Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, et al. (2013) Tumor and its microenvironment: A synergistic interplay. Semin Cancer Biol 23: 522-532.
- Yoshida S, Shime H, Matsumoto M, Kasahara M, Seya T (2019) Anti-oxidative amino acid L-ergothioneine modulates the tumor microenvironment to facilitate adjuvant vaccine immunotherapy. Front Immunol 10: 671.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3: 991-998.
- Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6: e1792.
Citation: Fukui-Miyazaki A, Tomaru U, Hirahashi T, Imai Y, Yoshida S, et al. (2020) A Hot-Water Extract of Spirulina platensis Enhances Spontaneous Cytotoxic T cell Activation in Mouse Tumor Models. J Mucosal Immunol Res 4:121.
Copyright: © 2020 Fukui-Miyazaki A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2938
- [From(publication date): 0-2020 - Nov 13, 2025]
- Breakdown by view type
- HTML page views: 2086
- PDF downloads: 852