A Game Changer in the personalised Treatment of Patients with Colorectal Cancer
Received: 10-Aug-2021 / Manuscript No. wjpt-20-17110 / Editor assigned: 12-Aug-2021 / PreQC No. wjpt-20-17110 / Reviewed: 03-Jan-2022 / QC No. wjpt-20-17110 / Revised: 08-Jan-2022 / Manuscript No. wjpt-20-17110 / Accepted Date: 14-Jan-2022 / Published Date: 15-Jan-2022 DOI: 10.4172/wjpt.1000144
Overexpression of fibroblast growth factor (FGF) in patients with colorectal cancer is usually associated with tumour development, however, there is fresh evidence for a tumour suppression role by FGF14. This dual activity opens new diagnostic opportunities for early screening of FGF biomarkers for the individualisation of therapies. Currently, there is a little known about FGFs and whether it will be worth implementing them as a prognostic tool for cancer treatment?
Colorectal cancer (CRC) is the second leading cause of cancer death in both sexes in the United States. Among sites of the digestive system, the colon and rectal cancers were estimated to cause 51,020 deaths in 2019 [1]. Unfortunately, the mechanism undelaying the development of cancer is extremely complex and heterogeneous, so early detection and use of ‘omics’ technologies are currently the best options to contribute to treatment success [2].
Patients with CRC have been reported to potentially overexpress most of the known FGFs at the late stages contributing to differentiation and proliferation of cancer cells [3-5]. Therefore, different approaches for blocking the FGF pathway with multikinase inhibitors and antibodies have been reported as targeted therapeutics [6]. To this day, there are 37 FDA approved kinase inhibitors, among which only one multikinase inhibitor for the metastatic colon cancer- Regorafenib that targets a number of tyrosine kinases including the FGF receptor 1 [7,8]. Such kinase therapies have shown unexpected toxicities that can be successfully avoided by applying a personalised approach with the help of cancer biomarkers [9]. Among antibody therapies, there are four monoclonal antibodies that bind to VEGF and EGFR are widely used in CRC in combination with chemotherapy or radiation therapy [10]. All these approaches are focused on direct inhibition of signalling pathways in CRC cells. However, recently published study by Su and colleagues [11] showed that supressed expression of FGF14 gene requires activation for the signalling inhibition in CRC.
FGF14 is an intracellular protein, a part of the fibroblast growth factor family which is localized on chromosome 13q33. By using a genome-wide screening approach, Su et al. [11] showed that FGF14 is silenced by promoter methylation and the treatment with approved DNA methyltransferase inhibitor 5-Aza suppressing the methylation process in CRC resulting in gene suppression. These findings suggest that modern epigenetics acting as methyltransferase inhibitors can effectively inhibit cancer cell viability through FGF activation.
Remarkably, FGF14 silencing or downregulation was observed in all CRC (10/10) cells exposing the idea that promoter methylation may be a predominant mechanism in the cancer development. In addition, to determine whether the restoration of the gene suppresses cell growth, Su and colleagues [11] performed a transfection study with the complementary DNA corresponding to the gene. The results confirmed that FGF14 gene expression promotes apoptosis of malignant cells. The mechanism behind this was determined to be induced via activation of death pathways by mitochondria. The cleavage of PARP, cleaved-caspase-3, cleaved-caspase-7 and Bax was observed by using the corresponding apoptotic markers. Knowing that impaired expression of this pathway is associated with colon tumorigenesis, Su et al. [11] assumed that upregulation of FGF14 can be used for cancer suppression. As expected, the results of Western blot analysis showed that upregulation of FGF14 resulting in inhibition of well-known signalling proteins PI3K, Akt and mTOR.
This important finding identified that activation of the FGF14 gene can potentially be a triple inhibitor of the most dysregulated PI3K/ AKT/mTOR signalling proteins in almost all human cancers including the colon. Novel inhibitors of this pathway are a highly attractive direction of today’s research. Only FDA approved drug suppressing PI3K is Idelalisib, whilst a number of drugs are employed in clinical trials [3,12]. Hence, the implementation of activation of FGF14 in combination with the receptor tyrosine inhibitors can potentially be an extremely beneficial therapy in 60-70% of CRC patients with mutations in PI3K/Akt [13].
In addition, Su and colleagues [11] confirmed the suppression role by FGF14 in vivo tumorigenicity study on nude mice transfected with the gene. The data obtained after 4 weeks of FGF14 injection showed a significant decrease in xenografted tumour volume (P<0.001). Thus, both studies on animal models and in vitro determined the importance of FGF14 as a promoter in CRC. So, instead of inhibiting all FGFs, clinicians should focus on first screening the genes expressed in abundance and then decide on whether to inhibit or activate FGFs [14].
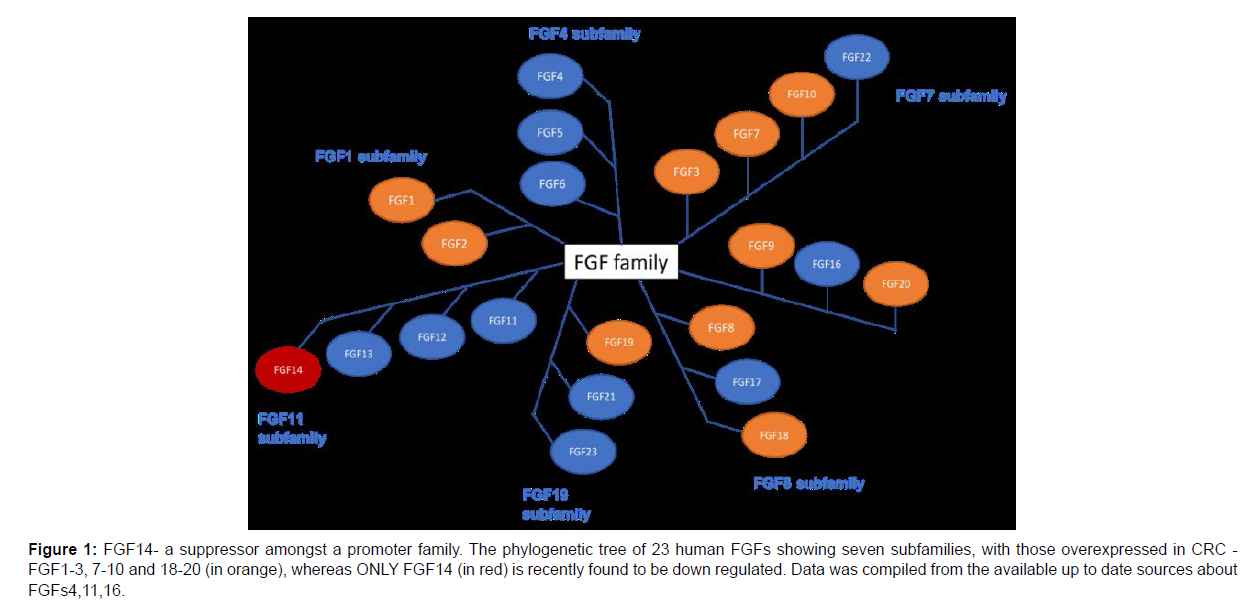
Su et al. [11] breakthrough of the first FGF suppressor gene in CRC among known FGFs indicates a need to find any other FGF suppressors, which can potentially reside among the same FGF11 subfamily (Fig.1). The mystery of a unique and diverse signalling system of FGF genes, receptors and their ligands may open eyes to a new wave of personalised therapies in colon cancer and many others. The field of CRC treatment is changing and maybe the screening of FGF genes in individuals would reframe current therapy approaches [2].
Independently, the findings by Matsuda et al. [4] and Qazvini et al. [3] studying FGF2 and FGF10 proteins in CRC suggested the use of anti-FGF receptor therapies to prevent the overexpression of those receptors and suppress tumour growth. So, methyltransferase inhibitors that can activate the FGF14 gene, in combination with the activators of FGF14 receptors raise a new perspective for treatment. Besides, the study by Jibiki et al. [15] showed that FGF levels tend to vary and progress in patients with advanced cancer, therefore the measurement of FGF genes expression may theoretically identify the clinical stage of CRC for the individualisation of the therapy.
Figure 1: FGF14- a suppressor amongst a promoter family. The phylogenetic tree of 23 human FGFs showing seven subfamilies, with those overexpressed in CRC - FGF1-3, 7-10 and 18-20 (in orange), whereas ONLY FGF14 (in red) is recently found to be down regulated. Data was compiled from the available up to date sources about FGFs4,11,16.
Currently, a panel of tests with the epigenetic component has been approved by the FDA to screen CRC patients’ stool and blood samples for the detection of mutated changes in the epigenome. The most commonly used test is FIT for haemoglobin detection in stools, other tests are focused on the analysis of the methylation level of genes BMP3, SEPT9 and CDC2 in stool or blood to predict the cancer stage. Additionally, another test involves using the biomarker miR-31-3p extracted from the primary tumours of the patients with colon cancer. Its results determine whether the individual will benefit from anti- EGFR or anti-VEGF therapy. Hence, there are successful commercially available epigenetic-based tests that can identify the stage of CRC and the therapy treatment [16]. So, the discovery of novel epigenetic biomarkers will further contribute to the precision medicine.
The remaining question is how soon we will be able to create FGF biomarkers for the CRC patients because there is not much known about the functions of each FGF gene among the subfamilies [5]. Although, some progress has been made in screening patients with FGF-21 marker for the identification of cardiometabolic disorder, and FGF-23 biomarker as a risk factor of cardiovascular, nonvascular diseases and cancer. However, this is usually done with additional markers associated with a particular disease, because FGF marker alone does not show a full picture of the pathophysiology of a disease [17]. Such markers are usually either CRC-relevant proteins or tumour antigens and antibodies against it [18]. Notably, the screening was performed with Olink PEA technology that enables measurement of only 92 proteins among which there are already FGF2,5,19,21,23. This exclusive technology is using antibodies specific for a target protein that has been detected by microfluidic qPCR. Despite the fact that there are few FGFs available, the company is focused on the extension of protein library, so FGF14 may be potentially screened in patients with colon cancer [19].
So, diagnostic tests for the identification of FGF genes are already available, it is just a matter of time when new FGF biomarkers will be used by clinicians [20]. Although the vast majority of tests are expensive and complicated to use, there is a future perspective that they will be simplified and cost-effective [16]. For now, research should focus on identification of the roles of all 23 FGFs and the molecules involved in its signalling pathway. Once biomarkers are discovered and validated, tests that are suitable for use by clinicians must be developed [21]. It seems like a lot of research needs to be done, but there is great promise that the development of successful biomarkers will improve precision medicine that will be conceivably used in clinical practice. Seeing that today’s medicines are on track to progress from a “one-size-fits-all” approach to personalized medicine confirms that biomarkers will play an important role in the future. Some of them are already offering the analysis of personal genome allowing detecting an effective treatment [20].
Certainly the creation of FGF markers will improve understanding of the patient’s disease progression, specifically for cancer, that remains the second most leading cause of death in the world. The early screening with FGF biomarkers would detect how aggressive cancer is and to which therapy the individual is most likely to respond well to. Biomarker’s usage already help us to understand the nature of the disease, so why not to continue to design novel markers and tests to deliver precision medicine?
References
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin. 69: 7-34.
- Aziz MA, Yousef Z, Saleh AM, Mohammad S, Al Knawy B (2017) Towards personalized medicine of colorectal cancer. Crit Rev Oncol Hematol 118: 70-78.
- Qazvini FF, Samadi N, Saffari M, Razavi ANE, Shirkoohi R (2019) Fibroblast growth factor-10 and epithelial mesenchymal transition in colorectal cancer. EXCLI J 18: 530-539.
- Matsuda Y, Ueda J, Ishiwata T (2012) Fibroblast growth factor receptor 2: expression, roles, and potential as a novel molecular target for colorectal cancer. Patholog Res Int 2012: 574768.
- Korc M, Friesel R (2009) The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 9: 639-651.
- Garcia AP, Barderas R, Torres S, Varas PH, Teixido J et al. (2013) FGFR4 role in epithelial- mesenchymal transition and its therapeutic value in colorectal cancer. PLoS One 8: e63695.
- García AM, Redondo M (2019) Targeting receptor kinases in colorectal cancer. Cancers 11: 433.
- Goel G (2018) Evolution of regorafenib from bench to bedside in colorectal cancer: Is it an attractive option or merely a “me too” drug? Cancer Manag Res 10: 425-437.
- Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A (2018) Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol Cancer 17: 48.
- https://www.cancerresearch.org/immunotherapy/cancer-types/colorectal-cancer
- Su T, Huang L, Zhang N, Peng S, Li X et al. (2020) FGF14 functions as a tumor suppressor through inhibiting PI3K/AKT/mTOR pathway in colorectal cancer. J Cancer 11: 819-825.
- Yang J, Nie J, Ma X, Wei Y, Peng Y et al. (2019) Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer 18.
- Pandurangan AK (2013) Potential targets for prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev 14: 2201-2205.
- https://www.cancer.net/cancer-types/colorectal-cancer/types-treatment
- Jibiki N, Saito N, Kameoka S, Kobayashi M (2014) Clinical significance of fibroblast growth factor (FGF) expression in colorectal cancer. Int Surg 99: 493-499.
- Garcia JB, Verdegal RO, Molla SM, Giménez JLG (2019) Epigenetic IVD tests for personalized precision medicine in cancer. Front Genet 10: 621.
- Sanders S, Oberst J (2017) Advancing precision medicine: Current and future proteogenomic strategies for biomarker discovery and development. Science/AAAS.
- Lech G, Slotwinski R, Slodkowski M, Krasnodebski IW (2016) Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol 22: 1745-1755.
- https://www.olink.com/data-you-can-trust/technology
- https://www.theguardian.com/science/2019/sep/28/genome-sequencing-precision-medicine-bespoke-healthcare-nhs
- Chaffey B, Silmon A (2016) Biomarkers in personalized medicine: Discovery and delivery. Biochem 38: 43-47.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Anna A (2022) A Game Changer in the Personalised Treatment of Patients with Colorectal Cancer. World J Pharmacol Toxicol 5: 144. DOI: 10.4172/wjpt.1000144
Copyright: © 2022 Anna A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2544
- [From(publication date): 0-2022 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 2068
- PDF downloads: 476