Research Article Open Access
A FDG-PET and fMRI Study on Glucose Metabolism and Hemodynamic Response during Visual Attentional Performance in Schizophrenia
| Jing Zhang1*, King-Wai Chu2, Erin A Hazlett2 and Monte S Buchsbaum2 | |
| 1Department of Bioinformatics, School of Biomedical Engineering, Capital Medical University, Beijing 100069, P.R. China | |
| 2Department of Psychiatry, Mount Sinai School of Medicine, New York, NY 10029, USA | |
| Corresponding Author : | Jing Zhang Department of Bioinformatics School of Biomedical Engineering Capital Medical University Beijing 100069, P.R. China Tel: +86-10-8391-1363 Fax: +86-10-8391-1544 E-mail: jzhang0000@163.com |
| Received September 17, 2013; Accepted October 11, 2013; Published October 17, 2013 | |
| Citation: Zhang J, Chu KW, Hazlett EA, Buchsbaum MS (2013) A FDG-PET and fMRI Study on Glucose Metabolism and Hemodynamic Response during Visual Attentional Performance in Schizophrenia. OMICS J Radiology 2:149. doi: 10.4172/2167-7964.1000149 | |
| Copyright: © 2013 Zhang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
This research investigated the correlation between regional cerebral glucose metabolism rate (rGMR) of [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) and hemodynamic response of blood-oxygen-leveldependent (BOLD) fMRI in schizophrenia during visual attentional performance. PET, MRI and fMRI images of 17 unmedicated schizophrenia patients and 38 healthy controls were acquired. A visual attention task was administered during the FDG uptake period and during the fMRI scan. Statistical parametric mapping (SPM) analysis was performed on the PET and fMRI images. Hemodynamic response curves were extracted from the frontal and temporal cortices, Anterior Cingulate Cortex (ACC), caudate and thalamus. The Area under the Curve (AUC) was calculated for each region and correlated with rGMR obtained from PET images. The results indicated that (1) Although the spatial patterns of rGMR and hemodynamic response SPMs are different, the rGMR and hemodynamic responses were significantly correlated in the frontal and temporal cortex, anterior cingulate cortex, caudate and thalamus for all subjects; (2) The patterns of the correlations are different between schizophrenia patients and controls. These results confirm the findings of our previous PET and fMRI study during auditory attentional performance, suggesting that altered blood flowmetabolic processes during neuronal activities may underlie the attention deficits in schizophrenia, which may be useful for the early diagnosis of schizophrenia.
| Keywords |
| Cerebral glucose metabolism; Hemodynamic response; Schizophrenia |
| Introduction |
| Early diagnosis and treatment is the key to good outcome and recovery of schizophrenia (Sz), a severe and disabling mental disorder. Considerable efforts have been made to identify potential biomarkers of schizophrenia in neuroimaging, genomics and psychophysiological measures, in order to support early diagnosis of schizophrenia. Structural imaging studies have reported reduced whole brain and hippocampal volumes [1], frontal-temporal gray matter volume loss [2], enlarged third and lateral ventricles [1,3], white matter abnormalities in the frontal and temporal lobe [4] in schizophrenia patients. Functional imaging studies have found that prefrontal function declines (“hypofrontality”) before the emergence of symptoms and frontostriatal function impaired thereafter [5], and decreased activation in the anterior cingulate [6]. There are other potential biomarkers such as a lower N-acetylaspartate/creatine ratio and dopamine overactivity identified in schizophrenia patients or high-risk people with Magnetic Resonance Spectroscopy (MRS), PET and Single Photon Emission Computed Tomography (SPECT) [1,3,7-9]. |
| Decreased Regional Glucose Metabolism (rGMR) in the prefrontal cortex and ACC in schizophrenia was found in FDGPET studies [1,3,10,11]. Interestingly, fMRI studies also reported decreased hemodynamic responses in the frontal cortex and ACC in schizophrenia patients during tasks [12,13]. These observations on hypofrontality (reduced metabolism or Cerebral Blood Flow (CBF) in the prefrontal cortex) using PET and fMRI trigger the following questions: What is the relationship between glucose metabolic and hemodynamic processes during neuronal activity? Are schizophrenia patients different from healthy controls in such metabolism-blood flow relationship? |
| It was believed that cerebral metabolism and CBF were tightly coupled in healthy subjects [14], but a mismatch in CBF and CMRO2 (cerebral oxygen metabolism rate) was found with PET using visual stimulation [15,16]. Then, non-linear flow-metabolism coupling was reported in a PET study in response to visual stimulation [17]. Pioneering work measuring CBF and metabolism in schizophrenia (with nitrous oxide) found no alteration in the overall average CBF and oxygen metabolism in patients [18], but it did not rule out neurophysiological abnormalities in specific brain regions in schizophrenia [19]. Traditionally, local rates of glucose utilization (LCMRglu) measured by the autoradiographic 2-deoxyglucose method (2-DG) [20,21] was determined following pharmacological treatments in schizophrenia [22], and regional CBF measured by Xenon-133 inhalation technique [23] was activated in schizophrenia patients during the Wisconsin card sorting task [24]. Recently, functional neuroimaging studies using PET, SPECT and fMRI have found that there is a time-dependent negative correlation between CBF and oxygen metabolism during visual stimulation [25-28], suggesting energy demands are met through anaerobic metabolism initially and require increased aerobic metabolism as stimulation continues [28]. In addition, spatial congruence was found in the activation maps of oxygen metabolism and hemodynamic response using H2(15)O PET and fMRI [29-31], and the sensitivity of fMRI can equal that of H2(15) O PET [32]. Further, a significant correlation between CBF, cerebral glucose concentration and oxygen metabolic rate was found in an animal model of ischemia [33]. |
| However, little is known about the relationship between glucose metabolism and CBF in patients with schizophrenia during cognitive tasks. In our previous research [34], this was studied using FDG-PET and BOLD fMRI during an auditory prepulse-to-attention task with a small sample (10 patients and 7 healthy controls). In this research, we further explored it with a larger sample using FDG-PET and BOLD fMRI during a visual attention task. |
| Materials and Methods |
| Subjects |
| 17 unmedicated schizophrenia patients (6 females, 11 males; age: 31.8 ± 10.4 years) were recruited for the spatial focus-of-attention study and 38 healthy subjects (15 females, 23 males; age: 30.5 ± 8.1 years) served as controls. All participants were screened for severe medical illness (such as diabetes), neurological illness (such as head trauma, central nervous system (CNS), neurological disease and seizure disorder) and substance abuse (within the past 6 months of entry into the study). They also had a negative urine toxicology screen and females a negative pregnancy test on scan day. All subjects were without metallic implants and eligible for MRI scan. They provided written informed consent in accordance with the Mount Sinai School of Medicine and James J. Peters Institutional Review Board guidelines. There were no significant differences in age and gender between the two groups. For detailed demographic characteristics of the subjects, Table 1 [35]. |
| FDG-PET data |
| Positron Emission Tomography (PET) scans (20 slices, 6.5-mm thickness) were obtained in the Neuroscience PET laboratory at the Mount Sinai Medical Center [36,37], with a head-dedicated GE scanner (model PC2048B) with measured resolution of 4.5 mm in plane (4.2-4.5 mm across 15 planes). T1-weighted axial MRI scans were acquired with the GE Sigma 5× system (TR=24 ms, TE=5 ms, flip angle=40°, slice thickness=1.2 mm, pixel matrix=256×256, field of view=23 cm, total slices=128). During the uptake period, the subjects performed a spatial focus-of-attention task and details of the task paradigm are described [38]. Briefly, the paradigm contained four runs and each lasted for 264 s. Each run began with a 24 s period of blank, the stimuli were presented at 2 s intervals (total stimulus block time=24 s), and there was a rest interval of 24 s afterward. During the task, the subjects clicked on a mouse button each time he detected the large letter target, ignored the flanking letters, and pressed on the right button for a right-sided target and the left button for a left-sided target. |
| fMRI data |
| Patients and controls in the study were scanned on a headdedicated Siemens Allegra 3T MRI scanner at MSMC. T1-weighted MP-RAGE (Magnetization Prepared Rapid Gradient Echo) imaging was acquired (208 slices with slice thickness=0.82 mm, matrix size=256×256×208, FOV=21 cm, TR=2500 ms, TE=4.38 ms, TI=1100 ms and an 8° flip angle FLASH acquisition) for high resolution structural images with good gray/white matter contrast. |
| Echoplanar images were acquired with a multi-slice 2D-EPI sequence (128×28 matrix, TR=2s, TE=40 ms, flip angle=90°, FOV=23 cm, slice thickness=5 mm, skip=2.5 mm), yielding 14 slices. BOLD fMRI acquisition occurred during a block-design visual spatial attention task. Details of the paradigm are described in (Buchsbaum et al. [38]). Briefly, there were 4 main stimuli or conditions in this paradigm: left hemifield-large letter, left hemifield-small display with flankers, right hemifield-large letter and right hemifield-small display with flankers. |
| Image processing and data analysis |
| Each PET image was preprocessed with non-brain removal using Brain Extraction Tool [39], and spatial smoothing using Gaussian profile filter of full-width-half-maximum (FWHM) 5 mm), and coregistered to the corresponding anatomical MRI data. SPM analysis was performed with FSL tools [40]. fMRI data were preprocessed with motion correction using MCFLIRT [41], non-brain removal using Brain Extraction Tool [39], spatial smoothing with Gaussian profile filter (FWHM=5 mm), and high-pass temporal filtering with Gaussian-weighted running line detrending (cutoff=70 s). fMRI images were co-registered to their structural MRI with a 7 Degrees-of- Freedom (DOF) linear transformation, followed by alignment to the MNI brain template using a 12 DOF linear fit. Since there are several conditions in the attention task of each study and fMRI SPM analysis separates the conditions while PET analysis not, the activations under separate conditions were combined in the SPM of fMRI in order to better match the SPM of PET images. |
| The Regions of Interests (ROIs), such as the caudate and thalamus, were traced on Anterior–Posterior Commissure (ACPC) positioned MRI. Brodmann Areas (BAs) in the frontal (BAs 9,11, 46, 47) and temporal (BAs 21, 22, 38) cortices, and anterior cingulate cortex (ACC) (BAs 24, 25, 32) were identified with Brodmann area analysis program developed in the NeuroScience PET Laboratory at the Mount Sinai School of Medicine. Details of the Brodmann area analysis approach were described [42,43], hemodynamic response curves were extracted from the frontal and temporal cortex, anterior cingulate, caudate and thalamus. Area under the curve (AUC) was calculated for each region (in each hemisphere, and/or for each tissue type) in each condition with the Root Mean Square (RMS) approach. The calculated AUC values were correlated with rGMR obtained from the PET image and the maximum correlation in each brain region (across hemispheres, tissue types and conditions) is considered as the correlation between glucose metabolism and hemodynamic response for that region. |
| Results |
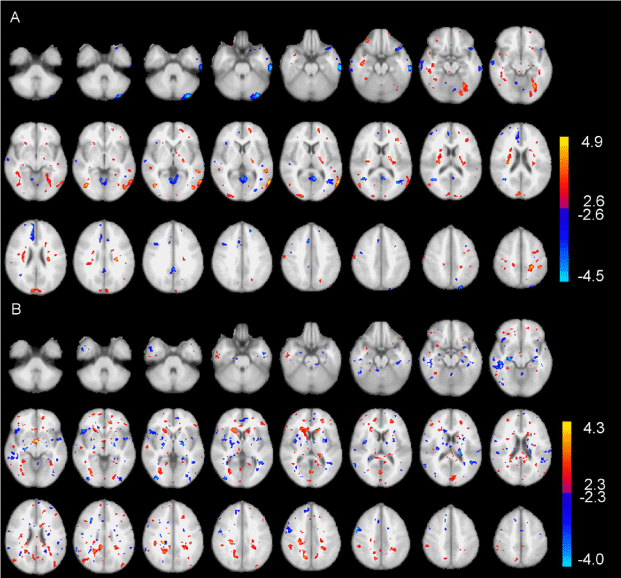
| The spatial patterns of the group SPM differences (Figure 1) are different, however, both rGMR and hemodynamic response are significantly (p<0.01) lower in patients than controls in regions, such as the frontal (BAs 9, 11, 46, 47) and temporal (BAs 21, 22, 38) cortices, and the ACC (BAs 24, 25, 32) (Table 1). |
| In addition, when considering all the subjects of this study for the correlation analysis, rGMR and hemodynamic response are significantly correlated in the frontal cortex (BAs 11, 47), temporal cortex (BAs 21, 22, 38), ACC (BAs 24, 25, 32), caudate and thalamus (Table 2). Further, as observed in our previous auditory attention study [34], the correlations between rGMR and hemodynamic response of patients in different regions are different from those of controls (Table 2). |
| Discussion |
| In this study, we investigated the correlation between rGMR and hemodynamic response during visual attentional performance in schizophrenia and found that although there were differences in the spatial patterns of the SPMs between rGMR and hemodynamic response, rGMR and hemodynamic response were significantly correlated in the frontal cortex (e.g. BA 11), temporal cortex (e.g. BA 21), ACC (e.g. BA 25), caudate and thalamus for all the subjects; and the correlation patterns of the patients were different from those of controls. These results are consistent with the findings in our previous study during auditory attention performance [34]. |
| Correlations between rGMR and hemodynamic responses |
| The SPMs of PET and fMRI images in this study demonstrated that patients had less hemodynamic responses and glucose metabolism in the fronto-temporal cortical regions than controls in response to the attention task. Such hypometabolism and low hemodynamic responses in schizophrenia patients in the frontal regions is consistent with the findings in PET studies [1,3,10,11] and fMRI studies [12,13] during tasks. The fronto-temporal hypometabolism and reduced hemodynamic responses found in this study revealed the functional deficits in the fronto-temporal regions in schizophrenia during attentional performance. Factors such as frontal-temporal gray matter volume loss [2], white matter abnormalities in the frontal and temporal lobe [44], altered dopaminergic and glutamatergic neurotransmission and disturbed cognitive processing in these regions may shed light on the mechanisms underlying such functional deficits [45]. |
| Compared with spatial congruence of the activations between oxygen metabolism (O15 water PET) and hemodynamic response (fMRI) [29,30,46], the spatial patterns in group SPMs of glucose metabolism and hemodynamic response are different (Figure 1), which indicates low spatial congruence. Such low spatial congruence in group spatial patterns may be due to the lack of baseline images (i.e., images taken at resting-state) in the FDG-PET data of this study (while the fMRI data, including both the baseline and activation images). Different inter-subject variations in PET and fMRI data may also contribute to the different spatial patterns of PET and fMRI SPMs. In an fMRI validation study using FDG-PET with both baseline and activation images, it was found that spatial congruence between PET and fMRI was relatively high for individual patients with mass lesion (such as tumor) in the central region of the brain, while the intersubject variations were also high [47]. In addition, the PET analysis results of this study reflect the end effects of several visual stimulation conditions, while fMRI analysis results reflect the combined effects of each single condition. Such difference may also lead to low spatial congruence between PET and fMRI in this study. |
| Although the rGMR and hemodynamic response are correlated in various brain regions, the signs and values of the correlations vary from one region to another. In addition, the correlations vary according to the tissue type (gray matter or white matter) of the ROI, the hemisphere of the ROI and the task performed (including different conditions of the task) (Table 2). Positive correlations reflect positive coupling of rGMR and flow (i.e. when CBF is increased in an ROI in a subject sample, rGMR also increases in the ROI of the sample). In contrast, negative correlations may reflect the opposite directions in which CBF and rGMR change. |
| It was found that oxygen metabolism was increased as brain activation lasted [48], and that rGMR was increased initially and decreased as stimulation continued, and there were matched changes in the blood flow and glucose utilization during brain stimulation [49]. MRS studies suggested that glycolysis is transient followed by an increase in oxygen utilization [50-52]. The correlations between glucose metabolism and blood flow might be explained by the observations of the matched changes in glucose utilization and blood flow over continuous brain stimulation, which is consistent with the hypothesis of glycolytically related blood flow regulation [49]. Further, a decrease in glycolysis with continuing visual stimulation may be associated with a decrease in the blood flow response through NADH/NAD+-related mechanisms [49]. |
| Different patterns of rGMR-flow correlations in patients and controls |
| The results of this study indicate that schizophrenia patients have different patterns from those of controls in the correlations of various regions between rGMR and hemodynamic responses (Table 2). This might be due to rGMR-flow uncoupling in regions, such as BAs 25 in patients with schizophrenia, but relatively matched glucose metabolism-flow coupling in regions, such as BA 25 in healthy controls. The reason for such different correlation patterns in patients and controls is unclear. Nevertheless, rGMR-flow uncoupling is common to a number of pathological disorders, such as brain trauma and epilepsy [53-55]. In addition, functional changes on PET, SPECT and fMRI in depressed patients include altered cerebral blood flow and metabolism in the pre-frontal cortex, ACC, caudate nucleus, amygdala and thalamus, suggesting abnormal interactions in several brain regions [53]. The increase in glucose utilization following activations caused by visual, auditory, olfactory, somatosensory or motor stimulations has been revealed in the pertinent brain structures [56]. Further, flow-metabolism uncoupling was found in traumatic brain injury (TBI) [54], and the pattern of flow-metabolism uncoupling obtained in TBI was similar to that in experimental head injury [57] and cerebral infarction [54,58,59] |
| Methodological issues |
| In this research, the correlation between glucose metabolism and cerebral blood flow was assessed with PET and fMRI, using global SPM analysis and local correlation analysis at each ROI. |
| As mentioned previously, one limitation of this study is that since PET images were acquired after the task was performed, PET analysis results reflect the end effects of all stimulation conditions, but fMRI analysis results reflect the combined effects of separate stimulation conditions, and thus the results of the correlation analysis may not fully reveal the true metabolism-flow relationship. An fMRI study with a task of only one visual stimulus may improve the PET and fMRI spacial congruence and correlation, and could be explored in our future studies. |
| Another limitation of this study is that we used AUC as an index for BOLD signal to calculate the correlations with rGMR for an ROI and used RMS approach to compute AUC (which was averaged across the voxels of the ROI). When the activations in the ROI are heterogeneous across the voxels, such averaged AUC approach might not accurately reflect the real changes of the BOLD signal within an ROI. An alternative approach is to obtain the Z-scores from SPM analysis and compare the local maxima Z-scores of the activated clusters in the ROI between rGMR and hemodynamic response. Such approach could be employed in the future, in order to better reveal the true metabolism-flow relationship. |
| Conclusion |
| In summary, the findings of this study are: (1) rGMR and hemodynamic responses are significantly correlated for all subjects in the frontal cortex (e.g. BA 11), temporal cortex (e.g. BA 21), anterior cingulate (e.g. BA 25), caudate and thalamus; (2) Schizophrenia patients have different patterns from those of controls in the correlations between rGMR and hemodynamic responses, which may suggest altered metabolism and blood flow processes underlying the attention deficits in schizophrenia. Since altered metabolism-flow processes often precede the emergence of major symptoms, the study of metabolism-flow relationship may be useful for the early diagnosis of schizophrenia. |
| Acknowledgement |
| We are grateful to the Department of Radiology at the Mt. Sinai Medical Center for their collaboration in image acquisition. This work is partly supported by NIH grant MH-60023 and NSF of China grant 81071211. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14464
- [From(publication date):
November-2013 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9833
- PDF downloads : 4631
