A District Level Linear Regression Analysis of Malaria Morbidity and Associated Control Interventions in Lusaka Province
Received: 11-Jan-2016 / Accepted Date: 11-Apr-2016 / Published Date: 18-Apr-2016 DOI: 10.4172/2161-1165.1000238
Abstract
Despite being a largely preventable and treatable disease, malaria is responsible for an estimated 50% of all infant mortality and 20% of all maternal mortality in Zambia and presents severe social and economic burdens on communities living in endemic areas. A retrospective, observational study was performed in Lusaka Province from 2009 to 2013. Provincial malaria surveillance data were analyzed using a district level linear regression model to explore the association between malaria morbidity and coverage with ITNs and IRHS. The study population included all patients who suffered from malaria in relation with the provincial population annually from January 2009 to December 2013. Multiple factor association between malaria morbidity and IRHS showed the P-value = 0.851 (p > 0.05) while malaria morbidity and ITN showed the P-value = 0.004 (p < 0.05). There was insignificant or no association between malaria morbidity and IRHS in Lusaka province from 2009-2013. However, there was a relatively strong association between malaria morbidity and ITN coverage. A district level linear regression analysis showed that there was insignificant association between malaria morbidity and IRHS but there was a relatively strong association between malaria morbidity and ITN coverage.
Keywords: Malaria morbidity; Malaria control interventions; Indoor residual house spraying; Insecticide treated nets; Lusaka province
165052Abbreviations
ACT: Atermisinin Combination Therapy; DEET: N,N-diethyl-3- methylbezamide; DHIS: District Health Information Software; DHO: District Health Office; IPT: Intermittent Preventive Therapy; IRHS: Indoor Residual House Spraying; ITN: Insecticide Treated Nets; LLIN: Long Lasting Insecticidal Nets; NMCC: National Malaria Control Center; RDT: Rapid Diagnostic Treatment
Introduction
Despite being a largely preventable and treatable disease, malaria is responsible for an estimated 800,000 deaths globally each year, with 90% of cases occurring in sub-Saharan Africa. In addition to its negative impact on health, malaria slows economic growth starting at individual level to the entire economies [1-3]. Zambia is not an exception where malaria has for a long time remained the leading cause of morbidity and mortality in both the children and adults. Although malaria affects the whole population, the most vulnerable are children under the age of 5 years and pregnant women [4].
Statistics also indicate that malaria accounts for up to 50% of all infant mortality and 20% of all maternal mortality in Zambia and presents severe social and economic burdens on communities living in endemic areas. In 2008, a total of 3.2 million cases of malaria (confirmed and unconfirmed) were reported countrywide with 3,871 deaths. However, malaria incidence per 1,000 population is reported to be on a steady decline from 412 cases in 2006 to 358 cases in 2007 and 252 cases in 2008 [4,5]. This is in response to calls for widespread control and elimination of malaria, where there has been a rapid scaleup of existing effective malaria control interventions in Zambia.
Malaria control and prevention entails the use of prophylactic drugs and other transmission-blocking tools such as indoor residual house spraying (IRHS), insecticide-treated mosquito nets (ITNs) including long-lasting insecticidal nets (LLINs) [6-9], intermittent preventive treatment (IPT) [10-12], artemisinin combination therapy (ACT) as first-line therapy coupled with efforts to improve access to prompt and effective treatment [13-15]. Effective management of malaria according to WHO has focused on insecticide treated nets (ITNs), indoor residual house spraying (IRHS) [6-9], intermittent presumptive therapy in pregnancy (IPTp) [10-12] and artemisinin-based combination therapy (ACT) [13]. Rapid diagnostic tests and treatment remains cardinal to all malaria suspected and confirmed cases respectively. However, other preventive measures such as environmental management, use of mosquito repellent and use of Personal Protection Clothes (PPC) are additional preventive measures especially in mosquito infested areas [16-18].
Despite the persistence of malaria in Zambia, different preventive and control strategies have been employed that have led to the decrease in malaria prevalence at national level although some areas still show high prevalence rates. The two primary interventions for vector control in Zambia are indoor residual house spraying (IRHS) and insecticidetreated nets (ITNs) [16-18].
This study aimed at establishing the relationship between malaria morbidity and associated control interventions in Lusaka Province of Zambia using malaria surveillance data, ITN and IRHS data from 2009 to 2013 through the Zambian District Health Information System (DHIS). The objectives were to establish the relationship between structures sprayed against mosquitoes and malaria morbidity from 2009 to 2013; and to show the relationship between ITN distribution and malaria morbidity from 2009 to 2013. Therefore, using malaria surveillance data collected from 2009 to 2013 through the Zambian District Health Information System, the burden of malaria and associated control interventions (ITNs and IRHS) in Lusaka province of Zambia were evaluated.
Materials and Method
Study location and population
Lusaka Province is located in the central part of Zambia and is the smallest province in the country with a surface area of 21,896 square kilometers.
The province shares internal borders with Central, Eastern and Southern provinces; and international borders with Mozambique and Zimbabwe in Luangwa district. Lusaka Province had an estimated population of 2,580,419 accounting for 17% of the country’s total population in the year 2013.
Although it is the smallest province, Lusaka is the most populated province in the country [19].
Administratively, the province is divided into eight districts namely, Chilanga, Chirundu, Chongwe, Kafue, Luangwa, Lusaka, Rufunsa and Shibuyunji. However, only the four original districts were considered in the study as new districts were still part of the four original districts at the beginning of the study.
Lusaka district, the capital city, is the smallest in terms of surface area (360 square kilometres), but had the largest population of 2,046,272 in 2013, thus accounting for 80% of total provincial population while Chongwe and Kafue districts had a population of 219,336 and 283,846 respectively.
The border towns of the capital city have a mix of peri-urban and rural zones. Luangwa district had the smallest provincial population of 30,965 inhabitants and is entirely rural [19,20]. Figure 1 shows thes location of Lusaka Province and its four original districts.
Study design
A retrospective, observational study was performed at each of the four original districts in Lusaka province of Zambia from 2009 to 2013.
Republic of Zambia Central Statistics Office (CSO) demographic data for Lusaka province were used to determine the study population as follows: (a) Annual district population to determine annual district malaria incidence and annual provincial population to determine annual provincial malaria incidence (where incidence = new cases / population × 1000). (b) Annual number of structures sprayed against mosquitoes per district to determine annual district IRHS coverage (number of people protected) and annual number of structures sprayed in the province to determine annual provincial IRHS coverage [where IRHS coverage = (structures sprayed against mosquitoes × 6) / population × 1000]. A factor 6 is the average number of people per household in Zambia. (c) Annual number of ITNs distributed per district to determine annual district ITN coverage and annual number of ITNs distributed in the province to determine annual provincial ITN coverage (where ITN coverage = ITNs distributed / population × 1000).
Data collection and analysis
Annual reported, district-level, aggregated malaria surveillance data, ITNs distribution and IRHS data were collected from all the four original districts of Lusaka Province from 2009 to 2013 using the Zambian District Health Information System (DHIS) through structured questionnaires.
5 questionnaires were used per district to capture data for a 5 year period. DHIS data are collected at health facilities (including district hospitals and health centers) in paper form and are sent to the District Health Office (DHO) for data capture and validation in the DHIS. The DHO transmits the district-level data to the provincial office for further processing and aggregation for the province.
The consolidated provincial data are then transmitted to the National Malaria Control Centre (NMCC) and Ministry of Health Headquarters, which in turn provide feedback and technical support to the provinces, districts and health facilities.
Data on annual provincial and district population were collected from Central Statistics Office (CSO) of the Republic of Zambia. District malaria surveillance data were analyzed using a district level linear regression model to explore the association between malaria morbidity and coverage with ITNs and IRHS.
The study population included all patients who suffered from malaria in Lusaka province in relation with the provincial population annually from January 2009 to December 2013. Variables were calculated as follows: dependent variables; malaria morbidity (malaria cases per thousand population), Independent variables; Indoor Residual House Spraying (IRHS) coverage per thousand households and Insecticide Treated Nets (ITNs) coverage per thousand population.
Results
Malaria control interventions
Lusaka Province IRHS coverage showed a gradual decrease from the baseline year 2009 to 2011. In 2009, Provincial IRHS coverage was 1,189 per 1,000 populations which went down to 1,121 in 2010 and decreased further to 1,053 in 2011. In 2012, IRHS was only conducted in isolated places and the coverage dropped to 280 per 1,000 populations which finally decreased to 35 per 1,000 populations in 2013 as only Chongwe District implemented the program during the year 2013.
ITN coverage in Lusaka Province was low during the study period. In 2009, 275 ITNs were distributed per 1,000 populations which went down to 247 in 2010 before further going down to 245 in 2011. There was however an increase in coverage during the year 2012 where 274 ITNs were distributed per 1,000 population. The year 2013 reported the highest coverage of 302 per 1,000 populations.
Single factor linear regression model
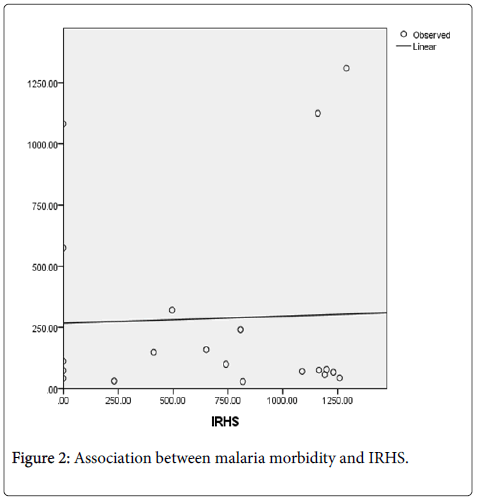
A district level linear regression model explored the association between malaria morbidity and IRHS. Figure 2 shows the scatter plot and linearity of the model.
Results from this single factor linear regression model showed that malaria morbidity and IRHS had a very weak or insignificant association with p-value of 0.882 (p > 0.05) from 2009 through to 2013 in Lusaka Province. Table 1 shows the results.
| B | SE | Beta | t | p-value | |
|---|---|---|---|---|---|
| IRHS Single factor model | |||||
| (Constant) | 267.016 | 159.442 | 1.675 | 0.111 | |
| IRHS | 0.028 | 0.189 | 0.035 | 0.15 | 0.882 |
Table 1: Association between malaria morbidity and IRHS coverage.
*Results significant at p < 0.05.
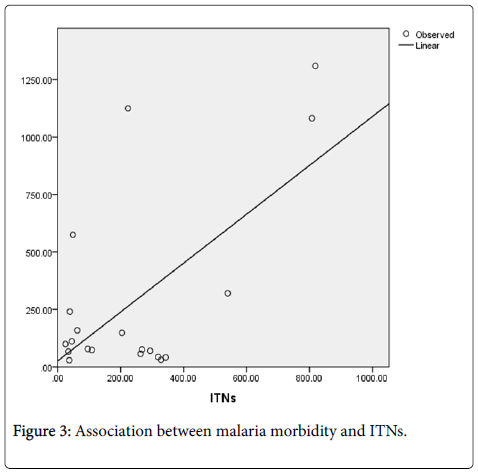
A district level linear regression model was also used to establish the association between malaria morbidity (dependent variable) and ITN coverage (independent variable) in Lusaka province from 2009 to 2013. These two variables were subjected to a curve estimation model (Figure 3) and scatter plot. Results of this model showed a relatively strong association between malaria morbidity and ITN coverage with p-value of 0.003 (p < 0.05). Table 2 shows the results.
| B | SE | Beta | t | p-value | |
|---|---|---|---|---|---|
| ITN Single factor model | |||||
| (Constant) | 25.824 | 104.38 | 0.247 | 0.807 | |
| ITN | 1.064 | 0.308 | 0.631 | 3.45 | 0.003 |
Table 2: Association between malaria morbidity and ITN coverage.
*Results significant at p < 0.05.
Multiple factor district level linear regression model
A multiple factor district level linear regression model explored the relationship between malaria morbidity (dependent variable) and associated control interventions (IRHS and ITN coverage) in Lusaka Province from 2009 to 2013. This multiple factor regression model showed a relatively strong association between malaria morbidity and ITN coverage with p-value of 0.004 (p < 0.05). The model also showed that there was a weak or insignificant association between malaria morbidity and IRHS with p-value of 0.851 (p > 0.05). Table 3 shows the results.
| B | SE | Beta | t | p-value | |
|---|---|---|---|---|---|
| Multiple factor model | |||||
| (Constant) | 6.051 | 149.1 | 0.041 | 0.968 | |
| IRHS | 0.029 | 0.151 | 0.036 | 0.191 | 0.851 |
| ITN | 1.064 | 0.317 | 0.631 | 3.357 | 0.004 |
Table 3: Relationship between malaria morbidity and associated control interventions.
*Results significant at p < 0.05.
Discussion
Malaria morbidity in Lusaka Province was 80 per 1,000 populations while IRHS coverage was 1,189 and ITN coverage was 275 per 1,000 populations during the baseline year 2009. Malaria morbidity increased steadily to 92 per 1,000 populations in 2011 while IRHS coverage decreased to 1,053 during the same period. ITN coverage also decreased steadily to 245 in 2011. From 2009 to 2011, malaria morbidity increased as a result of reduction in ITN coverage in Lusaka Province. However, ITN coverage increased from 2011 to 2013 which resulted in the decrease of malaria morbidity in the province from 80 to 60 per 1,000 of population.
A district level linear regression model explored the relationship between malaria morbidity and associated control interventions in Lusaka Province from 2009 to 2013. Results of the model indicated that there was insignificant or no association between malaria morbidity and IRHS. A single factor linear regression model showed the p-value of 0.882 (p > 0.05). However, the same model showed a relatively strong association between malaria morbidity and ITN coverage with the p-value of 0.003 (p < 0.05).
To further explore the association of IRHS and ITN coverage with malaria morbidity, a multiple factor linear regression model was applied. This model indicated similar results with single factor models. According to the multiple factor regression model, malaria morbidity and IRHS had insignificant or no association with p-value of 0.851 (p > 0.05). On the other hand, malaria morbidity and ITN coverage showed a relatively strong association with p-value of 0.004 (p < 0.05).
The failure of the model to establish a strong association between malaria morbidity and IRHS can be attributed to the fact that IRHS implementation was scaling down at the time when malaria morbidity was decreasing. IRHS has a potent period of 60 weeks which the model could not detect or establish. IRHS Chemicals remain effective on sprayed surfaces for a period of 60 weeks [21] after praying has been done. This implies that the spraying that was done from October to December 2011 remained effective even in 2012 when malaria morbidity went as low as 40 per 1,000 populations. A study on effectiveness or impact of IRHS on sprayed surfaces [21] which was conducted in Balai Ringin, Sarawak also supports this assertion.
On the other hand, the liner regression model explored and established a significant association between malaria morbidity and ITN coverage in Lusaka Province from 2009 to 2013. The reduction in malaria morbidity has been attributed to the increased ITN coverage in Lusaka Province especially from 2011 to 2013. These results are similar to a study done in Zambia where malaria morbidity showed an association with ITN coverage but not with IRHS [22]. Similar results were also obtained in Malawi in a cohort study where malaria morbidity was reduced following the scaling up of ITN coverage [23].
Despite a relatively strong association between malaria morbidity and ITN coverage, other malaria control interventions such as environmental management (filling water ponds with soil, grassslashing, etc.) and larviciding were also implemented during the study period. These control interventions could have some additional influence in reducing the prevalence of malaria but there was no standard data capturing system to help in measuring the additional impact. Results of this study have also shown that there is need to strengthen additional malaria prevention approaches to fight malaria scourge in Lusaka Province if malaria is to be eradicated. The traditional vector control interventions, such as insecticide-treated nets and indoor residual house spraying, protect the household but are less effective for individuals who are away from their homes during the peak times of vector feeding [24]. In these circumstances, topical repellents, such as N,N-diethyl-3-methylbenzamide (DEET), botanicals, citronella, picaridin, and olfactory binding proteins, could be viable methods to protect people who may be out of their homes [25].
Furthermore, decreased malaria infections have been associated with use of longlasting, insecticide-treated hammocks for forest workers in Vietnam, [26] insecticide-treated clothing such as chaddars and top sheets in refugee areas in Afghanistan [27] and insecticidetreated personal clothes and bedding in Kenya [28]. These approaches could be useful more especially in rural districts of Lusaka Province if malaria is to be eradicated.
This study had some limitations, only annual figures were collected per indicator. Both RDT and microscopy have limited sensitivity and specificity, and are particularly likely to misclassify individuals with low levels of parasitaemia. The observed associations between ITN and IRHS coverage and the burden of malaria were ecologic and not at the level of individuals. The study could not assess the impact of additional malaria control interventions such as larviciding due to lack of standard data capturing system.
Competing Interests
The authors declared that they have no competing interests.
Authors’ Contributions
MK designed the study, developed and programmed the model and drafted the manuscript. QL and GRS conceived the model and participated in the analysis. XFL conceived the study, participated in model development and review of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Compliments go to the Ministry of Health of the Republic of Zambia for allowing MK to conduct the study in Lusaka Province. MK is supported by Q Liu, GR Song and XF Li from the Department of Epidemiology and Biostatistics, School of Public Health, Dalian Medical University, Dalian 116044, Liaoning, PR China.
References
- Chima RI, Goodman CA, Mills A (2003) The economic impact of malaria in Africa: a critical review of the evidence. Health Policy 63:17-36.
- Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680-685.
- McPhee SJ (2006) An Introduction to Clinical Medicine (5th edn.) The McGraw-Hill Companies Inc, New York.
- Flaxman AD, Fullman N, Otten MW (2010) Rapid scaling up of insecticide-treated bed net coverage in Africa and its relationship with development assistance for health: A systematic synthesis of supply, distribution, and household survey data. Plos Medicine 7:e1000328.
- Noor AM, Mutheu JJ, Tatem AJ (2009) Insecticide-treated net coverage in Africa: mapping progress in 2000-07. Lancet 373: 58-67.
- Kilian A, Wijayanandana N, Ssekitoleeko J (2010) Review of delivery strategies for insecticide treated mosquito nets - are we ready for the next phase of malaria control efforts? TropIKA.
- Karema C, Aegawi M, Rukundo A (2012) Trends in malaria cases, hospital admissions and deaths following scale-up of anti-malaria interventions, 2000-2010, Rwanda. Malar J 11:236.
- Greenwood B (2006) Review: Intermittent preventive treatment-a new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Trop Med Int Health 11: 983-991.
- Aponte JJ, Schellenberg D, Egan A (2009) Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 374: 1533-1542.
- Pitt C,Ndiaye M, Patouillard E (2011) Economic report for the WHO Technical Expert Group Meeting on intermittent preventive treatment of malaria in children. LSHTM Research Online.
- Sinclair D, Zani B, Donegan S (2009)Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews. Cochrane Database Syst Rev 8: CD007483.
- Whitty CJM, Chandler C, Ansah E (2008) Deployment of ACT antimalarials for treatment of malaria: challenges and opportunities. Malar J 7: S7.
- Palmer KJ, Holliday SM, Brogden RN (1993) Mefloquine. Drugs 45:430-475.
- Bell D,Wongsrichanalai C, Barnwell JW (2006) Ensuring quality and access for malaria diagnosis: how can it be achieved? Nature Reviews Microbiology 4: 682-695.
- Reyburn H, Mbakilwa H, Mwangi R (2007) Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. British Medical Journal 334: 403.
- CDC (2014) Center for Global Health: Division of Parasitic Diseases and Malaria.
- (2012) Lusaka Provincial Health Office. Lusaka Province Annual Statistical Bulletin 2011. Provincial Health Office, Lusaka.
- Rohani A, Zamree I, Ali WNWM (2014) Impact of Indoor Residual- Sprayed Deltamethrin on different surfaces in a malaria endemic area in BalaiRingin, Sarawak. Advances in Entomology 2: 151-160.
- Kamuliwo M, Chanda E, Haque U (2013) The changing burden of malaria and association with vector control interventions in Zambia using district-level surveillance data, 2006-2011. Malaria Journal 12:437.
- Lindblade KA, Mwandama D, Mzilahowa T (2015) A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malaria Journal 14:31.
- Cotter C, Sturrock HJW, Hsiang MS (2013) The changing epidemiology of malaria elimination: new strategies for new challenges. The Lancet 382: 900-911.
- Katz TM, Miller JH, Hebert AA (2008) Insect repellents: historical perspectives and new developments. J Am AcadDermatol 58: 865-871.
- Erhart A, Thang ND, Hung NQ (2004) Forest malaria in Vietnam: a challenge for control.Am J Trop Med Hyg70:110-118.
- Rowland M, Durrani N, Hewitt S (1999) Permethrin-treated chaddars and top-sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans R SocTrop Med Hyg 93: 465-472.
- Kimani EW, Vulule JM, Kuria IW (2006) Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J 5: 63.
Citation: Kalubula M, Liu Q, Song GR, Li XF (2016) A District Level Linear Regression Analysis of Malaria Morbidity and Associated Control Interventions in Lusaka Province. Epidemiology (Sunnyvale) 6:238. DOI: 10.4172/2161-1165.1000238
Copyright: © 2016 Kalubula M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12966
- [From(publication date): 4-2016 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 11926
- PDF downloads: 1040