Short Commentary Open Access
A Different Role of Metallothionein-3 (Mt3) in Oxidative Stress and Neurodegeneration of Brain
Sook-Jeong Lee*
Department of New Drug Discovery and Development, Chungnam National University, Korea
- Corresponding Author:
- Sook-Jeong Lee
Department of New Drug Discovery and Development
Chungnam National University, 99 Daehak-ro, Yuseong, Daejeon 305-764, Korea
Tel: 82428217333
Fax: 82428218927
E-mail: sjlee0102@nate.com
Received date: June 16, 2016; Accepted date: June 26, 2016; Published date: June 30, 2016
Citation: Lee SJ (2016) A Different Role of Metallothionein-3 (Mt3) in Oxidative Stress and Neurodegeneration of Brain. J Neuroinfect Dis 7:221. doi:10.4172/2314-7326.1000221
Copyright: © 2016 Lee SJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Brain injury comes from various pathological conditions. Among those, acute brain injuries such as trauma and ischemia are mainly caused in the oxidative stress of brain cells. Labile zinc accumulation in the brain significantly contributes to oxidative brain injury. On the other hand, neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s (HD) are caused by progressive loss of structure or function of neurons. Different neurodegenerative disorders show many parallels including atypical protein assemblies as well as induced cell death
Introduction
Brain injury comes from various pathological conditions. Among those, acute brain injuries such as trauma and ischemia are mainly caused in the oxidative stress of brain cells [1-3]. Labile zinc accumulation in the brain significantly contributes to oxidative brain injury [4-8]. On the other hand, neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s (HD) are caused by progressive loss of structure or function of neurons. Different neurodegenerative disorders show many parallels including atypical protein assemblies as well as induced cell death [9,10]. Unlike the case of oxidative injury, zinc deficiency in the brain aggravates chronic neurodegeneration [11], suggesting that zinc dyshomeostasis plays a key role in the brain diseases.
Together with ubiquitin-proteasome pathway, autophagy-lysosome pathway has an important role in clearing of troublesome proteins and organelles [10]. Especially, zinc in lysosomes has a crucial role in autophagy pathway [11-13]. Metallothionein-3 (Mt3) is a zinc-binding protein enriched in the central nervous system (CNS) [14,15] and its deficiency also has a crucial role in the autophagy as well as amyloid beta (Aβ) endocytosis in the brain, thereby finally leading to AD as well as oxidative brain injury [11]. Here, we explain in detail how Mt3 is involved in two different brain injuries.
The Role of Mt3 in Oxidative Brain Injury
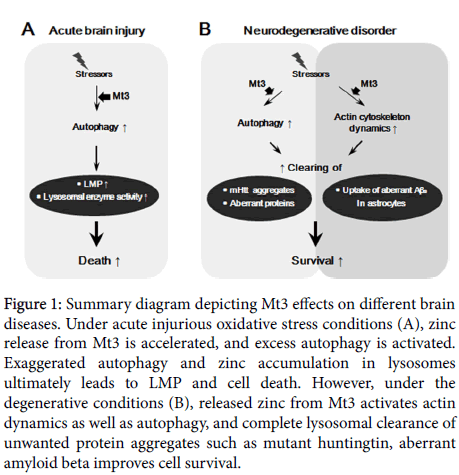
Zinc plays a major role in neuronal and glial oxidative injuries [5,16,17]. In peroxynitrite- and hydrogen peroxide (H2O2)-induced cell death, increases in free zinc levels induced p38 kinase activation and apoptosis [12,18,19]. Of many zinc sources, Mt3 is one of the major regulators of cellular zinc in the brain because this protein contains metal-cystein content in it and zinc has a high affinity for this protein. Therefore, Mt3 is able to accept or release zinc in response to changes in oxidative status [20,21] (Figure 1).
Figure 1: Summary diagram depicting Mt3 effects on different brain diseases. Under acute injurious oxidative stress conditions (A), zinc release from Mt3 is accelerated, and excess autophagy is activated. Exaggerated autophagy and zinc accumulation in lysosomes ultimately leads to LMP and cell death. However, under the degenerative conditions (B), released zinc from Mt3 activates actin dynamics as well as autophagy, and complete lysosomal clearance of unwanted protein aggregates such as mutant huntingtin, aberrant amyloid beta improves cell survival.
Except for its function as a zinc buffer, Mt3 may have more complex effects in the brain. The biological functions of Mt3 in the oxidative brain injuries may be from increased lysosomal enzyme activity and autophagy [8,12]. Various oxidative stressors initiate lysosomal membrane permeabilization (LMP), and thus a large amount of lysosomal enzymes containing proteases cathepsins are secreted from lysosomal lumen into cytosols. The secreted enzymes further activate caspases, finally leading to apoptotic cell death [8]. Besides, autophagic cell death mechanism has been recently proposed, as certain forms of cell death are attenuated by inhibition of autophagy [3]. A growing body of evidence shows that autophagic death contributes in acute brain injury [22,23]. A study with cultured model of oxidative cell injury revealed that Mt3 plays a key role in astrocytic cell death [12]. That is, Mt3-null mice presented altered mobility of lysosomal membrane protein 1 and reduced activity of lysosomal enzymes [12]. Abnormal function of lysosomes contributes to autophagy defect because lysosomes are the endpoint organelle in the autophagy pathway [24]. For this reason, Mt3-null condition may serve the beneficial effect on oxidative injury because the desensitization of lysosomal rupture and defect of autophagosome-lysosome fusion consequentially protect cells from apoptotic and autophagic cell death [8,12,16].
The Role of Mt3 in Neurodegenerative Disorders
Like two sides of the same coin, Mt3-null condition indicates a different effect depending on the type of encephalopathy. For instance, contrary to its positive effect in oxidative brain injury, knock-out of Mt3 negatively influences on neurodegenerative disease [25,26]. Degenerative brains show a decrease in the function of lysosome and autophagy, thus highly accumulating autophagosomes in diseased brains [27]. Unwanted proteins in cells disturb cell to cell communication in brain. Autophagy largely contributes to recycling of cellular proteins by clearing of unnecessary proteins and it partly serves smooth synaptic transmission in the brain. Mt3 level is downregulated in the AD brain [25,28] and Mt3-null astrocytes indicated low level of zinc in lysosome and autophagy defect [12]. Moreover, decrease in lysosomal zinc level contributes to mutant huntingtin (mHtt) aggregates in GFP-tagged mHtt polyglutamine (polyQ) expansion 74-transfected astrocytes [13], suggesting that cellular zinc plays a key role in degenerative brains.
Aβ is the main component of amyloid plaques and the most damaging form of Aβ may be oligomeric Aβs rather than the plaques themselves [29]. For this reason, misfolded oligomeric Aβs in extracellular space should be cleared by microglia and astrocytes because they block cell to cell signaling at synapses [30,31]. Thus, Aβs clearance has a key role in the pathologies of AD. Uptake of oligomeric Aβs in cultured astrocytes occurs mainly in a clathrin-dependent manner with a help of actin cytoskeleton, indicating that the role of actin is pretty important for endocytosis [11]. It has been recently found that disruption of actin cytoskeleton blocks Aβs endocytosis and the absence of Mt3 resulted in a defect in actin polymerization [32], thereby Aβs uptake in Mt3-null astrocytes noticeably decreased [11]. Therefore, AD and PD are accelerated by Mt3-null condition but etiological mechanisms are different.
Conclusion and Discussion
Deficiency in Mt3 may lead to two different changes in the brain, which are lysosomal biogenesis and cytoskeleton dynamics. Specifically, in the oxidative brain injury, knock-out of Mt3 may protect cells from oxidative damage because of desensitized lysosomal biogenesis and autophagy process. However, in the case of degenerative brain, dysfunction of lysosomes and actin cytoskeleton in Mt3-null astrocytes may contribute to accumulation of damaged proteins and toxic Aβs proteins. Except the function of Mt3 in lysosomes and cytoskeleton, metal-ion homeostasis by Mt3 may also play an important role in neurodegenerative diseases [33]. In PD, Cu(II) removal from the α-synuclein (α-Syn)-Cu(II) complex by thiolate ligands of Mt3 efficiently prevents α-Syn and dopamine oxidation, α-Syn oligomerization, and ROS formation [34].
Apart from the features of Mt3 described herein, Mt3 has other roles in the human diseases. Mt3 induction may serve as anti-inflammatory, anti-apoptotic agent and provides protection in cell therapy because zinc from Mt3 augments transcriptional regulation of genes involved in growth, cell proliferation, and differentiation [35]. Even though the role of Mt3 has not been clearly clarified yet and reported effects of Mt3 are not consistent, Mt3 may be closely associated with various cancers. In the bladder [36], breast [37,38], and prostate cancers [39], Mt3 expression was highly elevated and this alteration acted as a poor prognostic indicator. On the contrary, Mt3 level was downregulated in gastric carcinoma [40] and esophageal squamous cell carcinoma (EACs) [41]. In particular, it has been found that DNA methylation at -127 to -8 CpG sites of the promoter of xis essential for Mt3 mRNA expression. In addition, as significant hypermethylation at different sites within the promoter of Mt3 has been observed in EACs [42], aberrant patterns of DNA methylation may directly lead to the severe human disorders. As both the DNA methyltransferases (DNMTs) and some histone methyltransferases (HMTs) play a role in the establishment and maintenance of DNA methylation in mammals [43,44], inhibitors of these enzymes might serve as the novel therapeutic strategies for the Mt3 associated human diseases. Taken together, control of Mt3 function in human may offer hope for the therapeutic advances that could ameliorate many disease simultaneously.
Acknowledgements
This work was supported by the Chungnam National University.
References
- Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24: 133-150.
- Lewen A, Matz P, Chan PH (2000) Free radical pathways in CNS injury. J Neurotrauma 17:871-890.
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, et al. (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172: 454-469.
- Cho E, Hwang JJ, Han SH, Chung SJ, Koh JY, et al. (2010) Endogenous zinc mediates apoptotic programmed cell death in the developing brain. Neurotox Res 17: 156-166.
- Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY (2008)Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci 28: 3114-3122.
- Kim J, Kim TY, Hwang JJ, Lee JY, Shin JH, et al. (2009) Accumulation of labile zinc in neurons and astrocytes in the spinal cords of G93A SOD-1 transgenic mice. Neurobiol Dis 34: 221-229.
- Lai L, Zhao C, Su M, Li X, Liu X, et al. (2016) In vivo target bio-imaging of Alzheimer's disease by fluorescent zinc oxide nanoclusters. Biomater Sci 4: 1085-1091.
- Lee SJ, Seo BR, Choi EJ, Koh JY (2014) The role of reciprocal activation of cAbl and Mst1 in the oxidative death of cultured astrocytes. Glia 62: 639-648.
- Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443: 796-802.
- Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443: 780-786.
- Lee SJ, Seo BR, Koh JY (2015) Metallothionein-3 modulates the amyloid beta endocytosis of astrocytes through its effects on actin polymerization. Mol Brain 8: 84.
- Lee SJ, Park MH, Kim HJ, Koh JY (2010) Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia 58: 1186-1196.
- Park MH, Lee SJ, ByunHR, Kim Y, Oh YJ, et al. (2011) Clioquinol induces autophagy in cultured astrocytes and neurons by acting as a zinc ionophore. Neurobiol Dis 42: 242-251.
- Pedersen MO, Larsen A, Stoltenberg M, Penkowa M (2009) Cell death in the injured brain: roles of metallothioneins. ProgHistochemCytochem 44: 1-27.
- West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 29: 489-503.
- Lee SJ, Cho KS, Koh JY (2009) Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia 57: 1351-1361.
- Kimura T, Kambe T (2016) The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int J Mol Sci 17: 336.
- Aizenman E, Stout AK, Hartnett KA, DineleyKE, McLaughlin B, et al. (2000) Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem 75: 1878-1888.
- Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, et al. (2004)Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci 24: 10616-10627.
- Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130: 1455S-1458S.
- Maret W (2003) Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr 133: 1460S-1462S.
- Balduini W, Carloni S, Buonocore G (2009) Autophagy in hypoxia-ischemia induced brain injury: evidence and speculations. Autophagy 5: 221-223.
- Liu CL, Chen S, Dietrich D, Hu BR (2008) Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab 28: 674-683.
- Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, et al. (2012) Autophagy in lysosomal storage disorders. Autophagy 8: 719-730.
- Uchida Y (1994) Growth-inhibitory factor, metallothionein-like protein, and neurodegenerative diseases. Biol Signals 3: 211-215.
- Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein-like protein. Neuron 7: 337-347.
- Lee JA (2009) Autophagy in neurodegeneration: two sides of the same coin. BMB Rep 42: 324-330.
- Palmiter RD, Findley SD, Whitmore TE, Durnam DM (1992) MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci USA 89: 6333-6337.
- Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283: 29615-29619.
- Li C, Zhao R, Gao K, Wei Z, Yin MY, et al. (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr Alzheimer Res 8: 67-80.
- Zhao J, Paganini L, Mucke L, Gordon M, Refolo L, et al. (1996) Beta-secretase processing of the beta-amyloid precursor protein in transgenic mice is efficient in neurons but inefficient in astrocytes. J Biol Chem 271: 31407-31411.
- Lee SJ, Cho KS, Kim HN, Kim HJ, Koh JY (2011) Role of zinc metallothionein-3 (ZnMt3) in epidermal growth factor (EGF)-induced c-Abl protein activation and actin polymerization in cultured astrocytes. J Biol Chem 286: 40847-40856.
- Bonda DJ, Lee HG, Blair JA, Zhu X, Perry G, et al. (2011) Role of metal dyshomeostasis in Alzheimer's disease. Metallomics 3: 267-270.
- Meloni G, Vasak M (2011) Redox activity of alpha-synuclein-Cu is silenced by Zn(7)-metallothionein-3. Free Radic Biol Med 50: 1471-1479.
- Sharma S, Rais A, Sandhu R, Nel W, Ebadi M (2013) Clinical significance of metallothioneins in cell therapy and nanomedicine. Int J Nanomedicine 8: 1477-1488.
- Sens MA, Somji S, Lamm DL, Garrett SH, Slovinsky F, et al. (2000) Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environ Health Perspect 108: 413-418.
- Sens MA, Somji S, Garrett SH, Beall CL, Sens DA (2001) Metallothionein isoform 3 overexpression is associated with breast cancers having a poor prognosis. Am J Pathol 159: 21-26.
- Kmiecik AM, Pula B, Suchanski J, Olbromski M, Gomulkiewicz A, et al. (2015) Metallothionein-3 Increases Triple-Negative Breast Cancer Cell Invasiveness via Induction of Metalloproteinase Expression. PLoS One 10: e0124865.
- Garrett SH, Sens MA, Shukla D, Nestor S, Somji S, et al. (1999) Metallothionein isoform 3 expression in the human prostate and cancer-derived cell lines. Prostate 41: 196-202.
- Deng D, El-Rifai W, Ji J, Zhu B, Trampont P, et al. (2003) Hypermethylation of metallothionein-3 CpG island in gastric carcinoma. Carcinogenesis 24: 25-29.
- Smith E, Drew PA, Tian ZQ, De Young NJ, Liu JF, et al. (2005)Metallothionien 3 expression is frequently down-regulated in oesophageal squamous cell carcinoma by DNA methylation. Mol Cancer 4: 42.
- Peng D, Hu TL, Jiang A, Washington MK, Moskaluk CA, et al. (2011) Location-specific epigenetic regulation of the metallothionein 3 gene in esophageal adenocarcinomas. PLoS One 6: e22009.
- Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16: 6-21.
- Zhang T, Termanis A, Ozkan B, Bao XX, Culley J, et al. (2016) G9a/GLP Complex Maintains Imprinted DNA Methylation in Embryonic Stem Cells. Cell Rep 15: 77-85.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14793
- [From(publication date):
June-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 13637
- PDF downloads : 1156