A Comparison of the Viscoelastic Properties of Starch-polyacrylamide Graft Copolymers Produced in Dimethyl Sulfoxide and Water
Received: 21-Jul-2016 / Accepted Date: 14-Aug-2017 / Published Date: 21-Aug-2017
Abstract
The rheological properties of starch-polyacrylamide graft copolymers prepared in water and in dimethyl sulfoxide (DMSO) were investigated and compared. Both materials can absorb huge amount of water and form gels. Both water-made and DMSO-made starch-polyacrylamide graft copolymer gels exhibited viscoelastic solid properties. The analysis of modulus, concentration dependence, and stress relaxation measurements indicated that both water- made and DMSO-made starch-polyacrylamide gels were physical gels meaning that the cross-linkers between the molecules were of physical junctions. The linear range rheological property analysis suggested that water-made starch-polyacrylamide graft copolymers should be ‘weak’ gels at lower concentrations (<7%), but be ‘strong’ gels at higher concentrations (≥ 9%) however, the DMSO-made starch-polyacrylamide graft copolymers should be ‘weak’ gels at all measured concentrations. The non-linear steady shearing rheological properties studies showed that both water- made and DMSO-made starch-polyacrylamide graft copolymer gels exhibited shear thinning behaviour, which can be well fitted with the power law constitutive equation. The function and behaviour of both water-made and DMSO-made starch-polyacrylamide graft copolymer gels imply that these starch-based biomaterials can be potential candidates for applications in cosmetic and wound skin care gels; and the desired material’s behaviour and property can be manipulated by the copolymer’s concentration and preparation method such as in water or in DMSO.
Keywords: Biodegradable material; Graft copolymer; Polyacrylamide; Rheology; Starch; Viscoelastic properties
4243Introduction
Modified starch materials have attracted scientists’ interests since the starch-based super-absorbents were developed by USDA researchers [1,2]. Starch-based biopolymers can not only have similar functional behaviours as synthetic polymers, but also have much better environmental properties such as biodegradability. Various starch graft copolymers’ developments and preparations have been described and reported [3,4]. One of the starch-based graft copolymers is starch- polyacrylamide (PAAm). The preparation methods to produce starch- polyacrylamide (PAAm) graft copolymers include reactive blending [4-7] and reactive extrusion [8,9]. A variety of reactive blending processing in water medium to prepare starch-polyacrylamide (PAAm) graft copolymers has been reported using corn starch [5], cassava starch [6] and sago starch [7]. It has been well known that solvent selection can influence radical reactions [10,11]. Water and dimethyl sulfoxide (DMSO) are known to be good solvents for starch. Determining how solvent selection can impact the properties of the generated graft copolymers would be of value. However, no literature references can be found describing the preparation of starch-polyacrylamide (PAAm) graft copolymers in non-aqueous medium. The potential utilizations of modified starch-based materials include cosmetic or personal care gels, skin wound healing dressings, water treatment, paper manufacture and agricultural applications.
Recently, we prepared corn starch-polyacrylamide (PAAm) graft copolymers using a reactive blending method in both aqueous and dimethyl sulfoxide (DMSO) media. We found that both produced starch-polyacrylamide (PAAm) graft copolymers and could absorb more than one hundred times their weight in water and form gel- like materials. Since the properties of starch-PAAm graft copolymer prepared in DMSO have not been found in the literature; it is necessary to study the viscoelastic properties of starch-PAAm graft copolymer prepared in DMSO and to identify its potential usage. Thus, we explored and compared the viscoelastic properties of starch-PAAm graft copolymers prepared in water and in DMSO. The linear and non- linear viscoelastic properties for these materials are reported.
Materials and Methods
Materials.
The starch variety chosen for this research was Waxy 7350 (Tate and Lyle, Decatur, IL). Water was deionized and DMSO was from Sigma- Aldrich (St. Louis, MO).
Starch-polyacrylamide graft copolymer and polyacrylamide production
Starch was gelatinized in respective solvents by heating a starch suspension (6.5% solids) to 90°C and maintaining for 30 min. After gelatinization, the gelatinized starch solution was purged overnight with argon. Acrylamide (1.3 g) was added to the gelatinized starch solution, argon purge was continued for 30 min, while the acrylamide solubilized. Ammonium persulfate (0.53 ml of a 2% by mass solution) was added to initiate polymerization reaction. Samples were heated to 90°C for 2 h. Upon reaction, completion material was isolated by pouring the sample contents into 100% ethanol which quenched the reaction and resulted in precipitation of reaction products. The samples were then allowed to stir in ethanol for 20 min to remove any unreacted acrylamide monomer. The solid was recovered by filtration using Whatman 54 filter paper through a Buchner funnel. Solid was collected and allowed to stir in ethanol overnight. Product was again isolated by filtration. Collected solid was then dried in 105°C vacuum oven overnight and mass recovered was determined gravimetrically. Collected solid was analyzed for % nitrogen to determine conversion. Nitrogen contents were measured using a Perkin Elmer Series II CHNS/O analyzer 2400 (Perkin Elmer, Waltham MA) using acetanilide as a standard. GPC analysis was performed on separate aliquots where homopolymer was removed (30% ethanol/water extraction) and the starch was enzymatically removed [12]. Gel permeation chromatography (GPC) analysis was performed on a Shimadzu (Columbia, MD) GPC .The non-starch containing polyacrylamide polymers produced from water or DMSO were made in the same fashion, except for the subtraction of starch and the requisite gelatinization step. The water-made and DMSOmade starch-polyacrylamide graft copolymers as water-starch-PAAm and DMSO-starch-PAAm respectively were tested as after removal of residual monomer. The dry powder of water-starch-PAAm or DMSOstarch- PAAm graft copolymer was mixed with de-ionized water into the desired concentration and kept in 4°C. The sample was used within two days.
Measurements
A strain-controlled Rheometric ARES rheometer (TA Instruments, New Castle, DE) was used to perform the rheology studies [13]. The 50 mm diameter cone and plate as well as 25 mm parallel-plates geometries were adopted. The cone angle was 0.04 radians. The temperature was controlled at 25 ± 0.1°C by a water circulation system. The steady shear and stress relaxation experiments were also performed at 25 ± 0.1°C. Linear viscoelastic measurements were conducted for starch polymers. To ensure that all the measurements for the materials were made within the linear range for the linear viscoelastic properties studies, the strainsweep experiments were conducted initially. An applied shear strain valued in the linear range was adopted for the other viscoelastic property measurements for the same material; fresh samples were used for each experiment. Linear viscoelasticity indicates that the measured parameters are independent of applied shear strain. Small-amplitude oscillatory shear experiments were conducted over a frequency (ω) range of 0.1-500 rad/s, yielding the shear storage (G’) and loss (G”) moduli. The storage modulus represents the non-dissipative component of mechanical properties. The elastic or “rubber-like” behaviour is suggested if the G’ spectrum is independent of frequency and greater than the loss modulus over a certain range of frequency. The loss modulus represents the dissipative component of the mechanical properties and is characteristic of viscous flow. The phase shift or phase angle (δ) is defined by δ=tan-1 (G”/G’), and indicates whether a material is solid with perfect elasticity (δ=0) or liquid with pure viscosity (δ=90°) or something in between (0<δ<90°). Stress relaxation experiments measured the stress relaxation during the time after the material is subject to a step-increase in shear strain.
Results and Discussion
The percent conversion for the two systems was determined by measuring the % nitrogen in the solid after removal of monomer using Equation 1, where M is the solid recovered (after monomer extraction), N1 is the % nitrogen of the solid recovered, 17.49 is the theoretical %N for polyacrylamide (using an average of lab produced and purchased polyacrylamide) and A1 is the amount of acrylamide used. The waterstarch- PAAm had ~100% conversion, while the DMSO-starch-PAAm had 80% conversion. For the homopolymers of acrylamide made in either water or DMSO, the % conversion was determined by mass after removal of monomer. The water-PAAm had 89% conversion and the DMSO-PAAm had 83% conversion. The graft content is a ratio of the % nitrogen for the starch-PAAm (after extraction to remove monomer) and the % nitrogen for polyacrylamide. The graft content (GC) for water-starch-PAAm and DMSO-starch-PAAm the GC was 44% and 38% respectively. By using Equation 2, it can be determined on average how many anhydro-glucose units (AGU) are present between polyacrylamide grafts. In Equation 2, GC is the graft content, 162 is the molecular weight for AGU and Mn is the number average molecular weight of the polymer. For water-starch-PAAm and DMSO-starch- PAAm the average number of AGU in between grafts was 364 and 87 respectively. There were 4x more grafts present on the starch when the graft radical polymerization was carried out in DMSO versus water.
% conversion=(M*N1)/(A1*17.49) (1)
AGU between grafts=(100-GC)/162)/(GC/(Mn/2) (2)
The weight average molecular weight (Mw) of the water-PAAm was 410,000 and the number average molecular weight (Mn) was 92,600. The Mw of the DMSO-PAAm was 39,000 and the Mn was 17,300. The DMSO solvent had a large impact on the molecular weight of the polyacrylamide. The molecular weight of the grafted polyacrylamide chains was determined after removal of the starch using enzymatic hydrolysis. The completion of starch hydrolysis was confirmed by thin layer chromatography and Lugol’s iodine [8,9,12]. By doing this, the size and frequency of the grafts can be determined. For the waterstarch- PAAm the Mw was 411,000 and the Mn was 92,800. For the DMSO-starch-PAAm the Mw was 39,300 and the Mn was 17,300. Additional work is in progress detailing how reaction conditions affect the architecture and describing the mechanism of why the solvent impacts the architecture of the starch-graft-polyacrylamides.
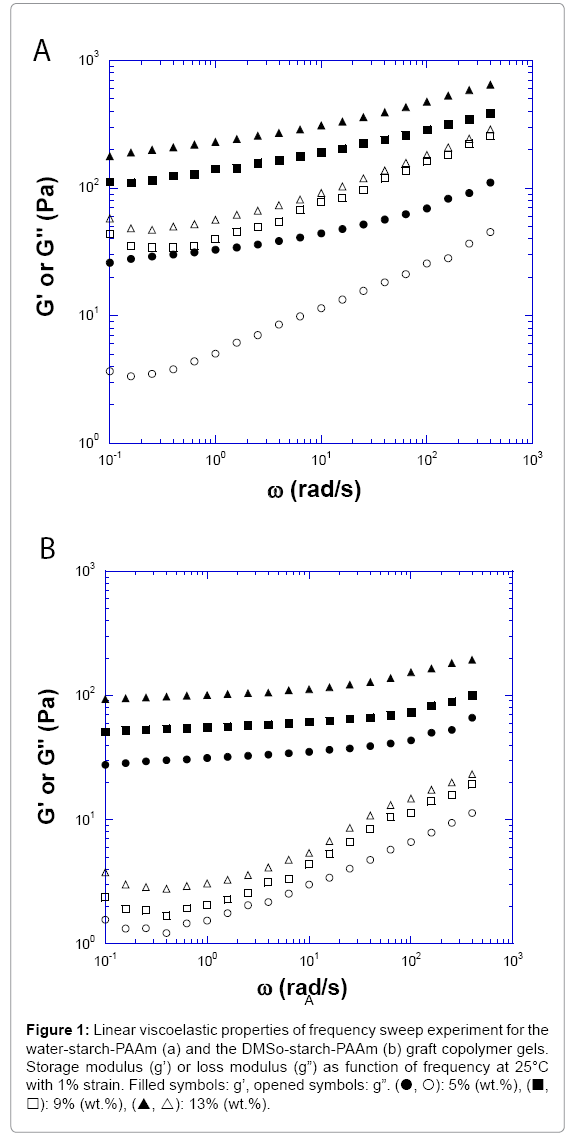
It is well known that at room temperature, un-modified starches have low solubility in water and will not gelatinize. However, the graft copolymer starch-PAAm will absorb hundreds of times their weight in water and form gels at room temperature. Five concentrations of watermade and DMSO-made starch-PAAm gels from 5% to 13% (wt %) at 25°C were investigated. After the powder of starch-PAAm graft copolymer mixed with water at room temperature, a gel-like material formed. At 25°C, all measured concentrations of both water-starch-PAAm and DMSO-starch-PAAm graft copolymer samples exhibited viscoelastic solid properties. The storage moduli (G’) were all greater than loss moduli (G”) at the measured frequency range (Figure 1). The linear viscoelastic properties of the water-starch-PAAm copolymer gels were concentration dependent; the higher the concentration of the water-starch-PAAm, the greater its viscoelastic modulus (Figure 1a). For the 5% (wt.%) waterstarch- PAAm gel, the storage modulus (G’) at 1 rad/s frequency was about 33 Pa. The phase shifts for the 5% water-starch-PAAm gel were in the range of 6.9°C-22.3°C. For the higher concentration of 13% (wt.%) waterstarch- PAAm gel, the storage modulus (G’) at 1 rad/s frequency became about 230 Pa, an increase of about seven times. While the phase shifts for the 13% water-starch-PAAm gel were in the range of 7.7°C-23.9°C which were similar to those for the 5% water-starch-PAAm. The storage (G’) moduli curves for the water-starch-PAAm gels were slightly frequency dependent; while the curves of loss moduli (G”) were more frequency dependent than that of storage moduli (G’) (Figure 1a). The phase shifts for all measured water-starch-PAAm copolymer gels were in the range of 6.9°C-32.9°C. The linear rheological properties of the DMSO-starch- PAAm copolymer gels were also concentration dependent; the higher of the concentration of the DMSO-starch-PAAm, the higher its viscoelastic modulus (Figure 1b). For the 5% (wt %) DMSO-starch-PAAm gel, the storage modulus (G’) at 1 rad/s frequency was about 31 Pa, which was similar to that of the water-starch-PAAm gel. The phase shifts for the 5% DMSO-starch-PAAm gel were in the range of 2.3°C to10.1°C. For the higher concentration of 13% (wt. %) DMSO-starch-PAAm gel, the storage modulus (G’) at 1 rad/s frequency became about 100 Pa, an increase of more than three times. However, this was one half that of the waterstarch- PAAm gel. While the phase shifts for the 13% DMSO-starch- PAAm gel were in the range of 1.6o to 6.8°, which were smaller than those for the 5% DMSO-starch-PAAm. The storage (G’) moduli curves for the DMSO-starch-PAAm gels were almost independent of frequency while the curves of loss moduli (G”) were slightly frequency dependent (Figure 1b). These curve shapes were similar to those for rubber-like gel materials [14]. Comparing the moduli curve shapes for the DMSO-starch-PAAm and water-starch-PAAm gels, the water-starch-PAAm gels exhibited relatively more fluid-like behaviour than the DMSO-starch-PAAm gels. With the same concentration, the moduli values for the DMSO-starch- PAAm gels were similar but lower than those for the water-starch-PAAm gels. This implied that DMSO-starch-PAAm should have smaller average molecular weight or size compared with water-starch-PAAm considering the same material with the same concentration only in different media (DMSO and water). This result is consistent with the molecular weight stated earlier in this report. The phase shifts for all measured DMSO- starch-PAAm copolymer gels were in the range of 1.0°C-10.1°C, which were much smaller than those for the water-starch-PAAm. This result indicated that the DMSO-starch-PAAm copolymer was more solid- like than water-starch-PAAm. The linear viscoelastic properties shown above indicated that water-starch-PAAm and DMSO-starch-PAAm graft copolymers exhibited different behaviours, which suggested that water-starch-PAAm and DMSO-starch-PAAm should have a structural difference. The rational explanation is that DMSO-starch-PAAm contains many short chains of polyacrylamide, but water-starch-PAAm contains fewer long chains of polyacrylamide at the same concentration. Many short polyacrylamide chains and starch chains of the DMSO-starch- PAAm should have more chain-chain interaction contact points or physical cross-linking knobs compare to the water-starch-PAAm; while water-starch-PAAm should have fewer chain-chain physical interaction contact points or physical cross-linking knobs due to having fewer long polyacrylamide chains. Therefore, the DMSO-starch-PAAm copolymer gels exhibited more solid-like behaviour with the smaller phase shifts.
Figure 1: Linear viscoelastic properties of frequency sweep experiment for the water-starch-PAAm (a) and the DMSo-starch-PAAm (b) graft copolymer gels. Storage modulus (g’) or loss modulus (g”) as function of frequency at 25°C with 1% strain. Filled symbols: g’, opened symbols: g”. (?, ?): 5% (wt.%), (?, ?): 9% (wt.%), (?, ?): 13% (wt.%).
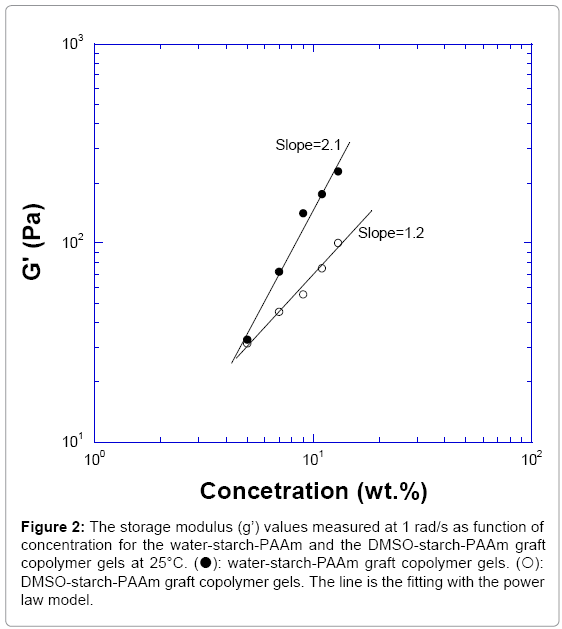
The trend of value of log (G’) versus log (C) for both water-starch- PAAm and DMSO-starch-PAAm copolymers followed the power law model described for physical gels [15]. The modulus of water-starch- PAAm gel varied as C2.1; while the modulus of DMSO-starch-PAAm gel varied as C1.2 (Figure 2). The power law index is dependent on the kinetics that the gel formed [15]. These results supported the fact that both of the water-starch-PAAm and DMSO-starch-PAAm gels were physical gels and the measured concentrations were in the range of near critical concentration since their power index number were around two. This result supported that the chain-chain interactions or cross-linking for both water-starch-PAAm and DMSO-starch-PAAm were physical interactions instead of chemical or covalent cross-linking.
Figure 2: The storage modulus (g’) values measured at 1 rad/s as function of concentration for the water-starch-PAAm and the DMSO-starch-PAAm graft copolymer gels at 25°C. (?): water-starch-PAAm graft copolymer gels. (?): DMSO-starch-PAAm graft copolymer gels. The line is the fitting with the power law model.
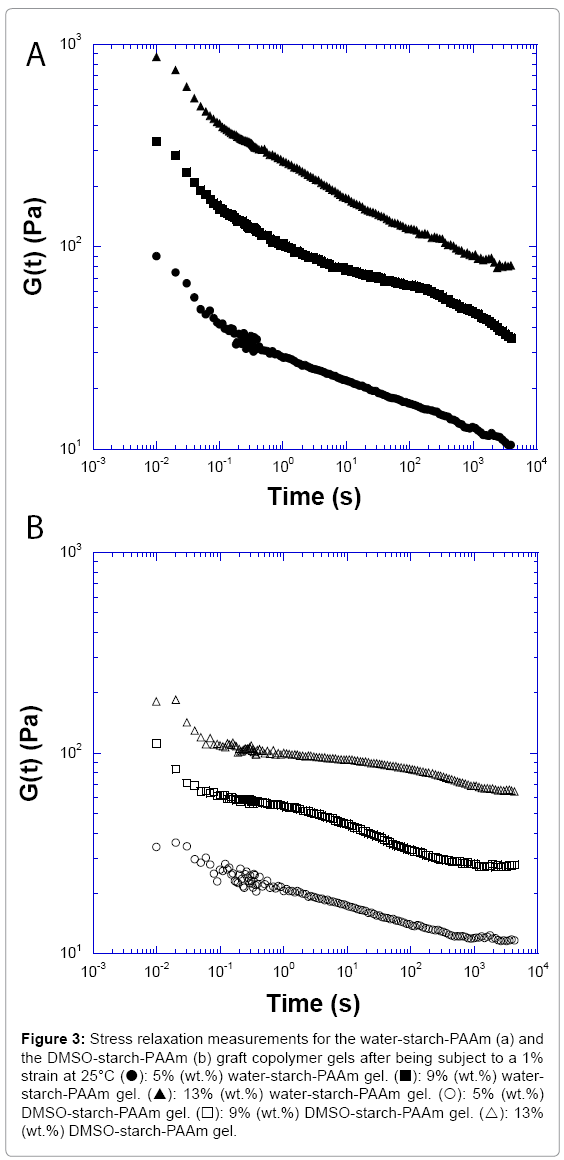
To further explore the evidence of whether the water-starch-PAAm and DMSO-starch-PAAm copolymer gels are physical gels or chemically cross-linked networks, we conducted stress relaxation measurements.
The stress relaxation measurements for the water-starch-PAAm and DMSO-starch-PAAm copolymer gels are shown in Figure 3. The stress relaxation experiment showed that both water-starch-PAAm and DMSO-starch-PAAm copolymer gels had a long relaxation time. They were not fully relaxed after more than 3000 s. This result supported again that both water-starch-PAAm and DMSO-starch-PAAm copolymers are physically cross-linked gels, not chemically cross-linked ones even though they have long relaxation times. Because if a network is tightly cross-linked chemically, there should rarely be seen any relaxation, and relaxation time should be infinite. This result is also consistent with the above conclusion that both water-starch-PAAm and DMSO- starch-PAAm copolymers are physical gels that the power law model predicted. Comparing two copolymer gels relaxation rate, we can see that the water-starch-PAAm gels clearly relaxed faster than the DMSO- starch-PAAm gels (Figure 3).The reason can again be explained by the fact that the DMSO-starch-PAAm gel had more physical cross-linkers due to it having many short polyacrylamide chains versus few long chains in the water-starch-PAAm gel. Thus, the DMSO-starch-PAAm gel was relatively more rigid and more difficult to relax, while the water- starch-PAAm gel had fewer physical cross-linkers due to containing fewer long polyacrylamide chains. Thus, the long chains of the water- starch-PAAm gel will be relatively easy to diffuse by each other and easier to relax.
Figure 3: Stress relaxation measurements for the water-starch-PAAm (a) and the DMSO-starch-PAAm (b) graft copolymer gels after being subject to a 1% strain at 25°C (?): 5% (wt.%) water-starch-PAAm gel. (?): 9% (wt.%) water- starch-PAAm gel. (?): 13% (wt.%) water-starch-PAAm gel. (?): 5% (wt.%) DMSO-starch-PAAm gel. (?): 9% (wt.%) DMSO-starch-PAAm gel. (?): 13% (wt.%) DMSO-starch-PAAm gel.
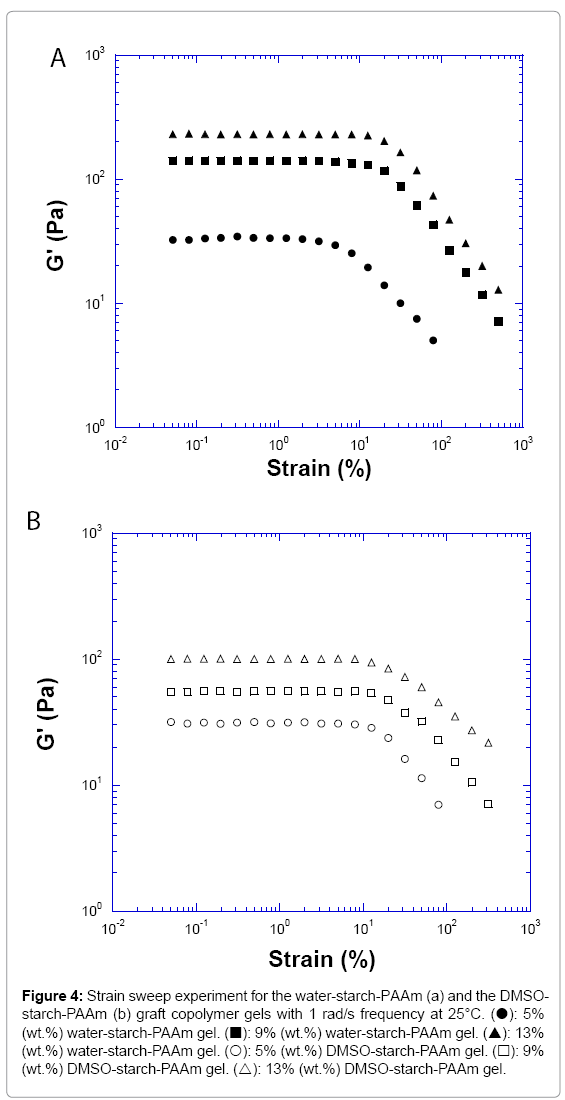
The strain sweep measurements of three concentrations of water- starch-PAAm and DMSO-starch-PAAm copolymers gels are presented in Figure 4. The linear range of 5% and 7% (data not shown for the 7% sample) of the water-starch-PAAm gels were small, around 10% of the shear strain; while the linear range of >9% of the water-starch- PAAm gels increased to about 20% (Figure 4a). The linear range of all measured concentrations of the DMSO-starch-PAAm copolymers was small, around 10% of the shear strain (Figure 4b). These results indicated that the water-starch-PAAm gels were networks - so called “weak” gels [15] at lower concentrations, but became so called “strong” gels [15] at higher concentrations. The DMSO-starch-PAAm copolymers were “weak” gels at all measured concentrations. A “weak” gel is cross-linked together by linkages that are relatively stronger and more permanent than entanglements. While a gel formed by chain- chain entanglement is the characteristics of a “strong” gel. However, a “weak” gel is extremely susceptible to disruption, while an entangled “strong” gel can have a linear range to 20% or more. Cross-linkers can be classified as covalent or physical junctions. For the water-starch- PAAm copolymers, the chain-chain entanglements were not strong enough at the lower concentrations due to dilution, so the networks were “weak” gels. However, the water-starch-PAAm copolymers’ chain-chain entanglements became stronger and stronger with the increasing concentration; thus the networks were “strong” gels at higher concentrations. For the DMSO-starch-PAAm copolymers, the networks were dominated by the physical cross-linking by the short chains; therefore they were “weak” gels at all measured concentrations. The linear rheological properties that we investigated above for both water-starch-PAAm and DMSO-starch-PAAm copolymers gels are similar to those for some of the cosmetic gels [16-18] and wound healing materials [19-22]. The behaviour of the gel could be manipulated and controlled by concentration, preparations with water or DMSO.
Figure 4: Strain sweep experiment for the water-starch-PAAm (a) and the DMSO- starch-PAAm (b) graft copolymer gels with 1 rad/s frequency at 25°C. (?): 5% (wt.%) water-starch-PAAm gel. (?): 9% (wt.%) water-starch-PAAm gel. (?): 13% (wt.%) water-starch-PAAm gel. (?): 5% (wt.%) DMSO-starch-PAAm gel. (?): 9% (wt.%) DMSO-starch-PAAm gel. (?): 13% (wt.%) DMSO-starch-PAAm gel.
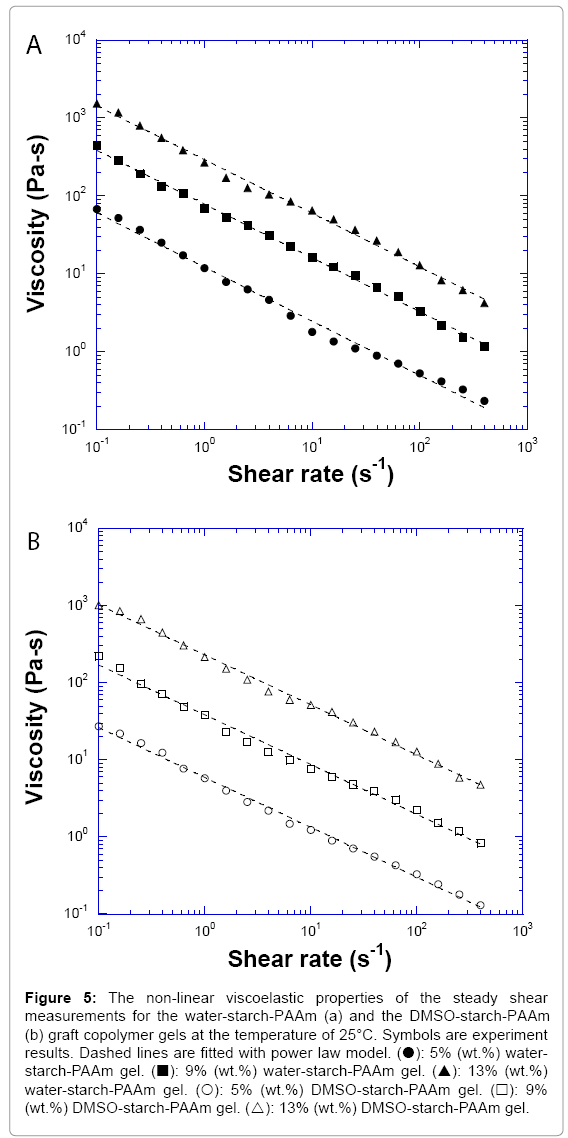
In order to better understand the processing behaviour, the non- linear steady shear viscoelastic properties for both water-starch-PAAm and DMSO-starch-PAAm copolymer gels were studied. All of the studied materials exhibited shear-thinning behaviour over the entire measured shear rates (Figure 5). Shear-thinning rheological behaviour can be characterized by a power law constitutive equation [23]. The power law equation may be written as
Figure 5: The non-linear viscoelastic properties of the steady shear measurements for the water-starch-PAAm (a) and the DMSO-starch-PAAm (b) graft copolymer gels at the temperature of 25°C. Symbols are experiment results. Dashed lines are fitted with power law model. (?): 5% (wt.%) waterstarch- PAAm gel. (?): 9% (wt.%) water-starch-PAAm gel. (?): 13% (wt.%) water-starch-PAAm gel. (?): 5% (wt.%) DMSO-starch-PAAm gel. (?): 9% (wt.%) DMSO-starch-PAAm gel. (?): 13% (wt.%) DMSO-starch-PAAm gel.
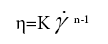
where η is the shear viscosity, K is the front factor, γ is the shear rate, and n is the power law exponent. The value of n is less than one for material shear-thinning behaviour. We used Equation 3 to fit shear- thinning viscosity for all measured samples. The experimental data were very well fitted by the power law constitutive equation (Figures 5a and 5b). The results of the fits are summarized in Table 1. From Table 1, we can get the impression that water-starch-PAAm and DMSO-starch- PAAm copolymer gels possess almost the same power law exponent of 0.31 and 0.35, respectively. Therefore, water-starch-PAAm and DMSO-starch-PAAm copolymer gels should have very similar shear-thinning extent. These results suggested that water-starch-PAAm and DMSO-starch-PAAm copolymer gels should have similar behaviour during processing. The viscosities of both water-starch-PAAm and DMSO-starch-PAAm copolymer gels were concentration dependent; the greater the concentration, the higher the viscosities (Figure 5). The non-linear rheological properties that we investigated above for both water-starch-PAAm and DMSO-starch-PAAm copolymer gels are also similar to those for some of the cosmetic gels [16-18] and wound healing materials [19-22]. Thus, both linear and non-linear viscoelastic behaviours studies in this work suggest that water-starch-PAAm and DMSO-starch-PAAm copolymer gels should be good candidates for cosmetic and wound healing materials.
| Material | K (Pa-sn) | n | R2 |
|---|---|---|---|
| 5% (wt.%) water-starch-PAAm gel | 12.2 | 0.31 | 0.99 |
| 7% (wt.%) water-starch-PAAm gel | 49.5 | 0.31 | 0.95 |
| 9% (wt.%) water-starch-PAAm gel | 78.6 | 0.31 | 0.99 |
| 11% (wt.%) water-starch-PAAm gel | 132.5 | 0.31 | 0.99 |
| 13% (wt.%) water-starch-PAAm gel | 293.2 | 0.31 | 0.99 |
| 5% (wt.%) DMSO-starch-PAAm gel | 5.9 | 0.35 | 0.99 |
| 7% (wt.%) DMSO-starch-PAAm gel | 19.5 | 0.35 | 0.99 |
| 9% (wt.%) DMSO-starch-PAAm gel | 38.4 | 0.35 | 0.99 |
| 11% (wt.%) DMSO-starch-PAAm gel | 67.2 | 0.35 | 0.96 |
| 13% (wt.%) DMSO-starch-PAAm gel | 230.5 | 0.35 | 0.99 |
Table 1: Power law model fitted parameters for the non-linear viscoelastic properties of the water-starch-PAAm and DMSO-starch-PAAm graft copolymer gels.
Conclusion
The linear and non-linear rheological properties for water-starch- PAAm and DMSO-starch-PAAm copolymer gels were investigated. Both materials exhibited viscoelastic solid behaviours; and their properties could be manipulated by concentration and preparation method. Both kinds of gels were physical gels, which followed the physical gel power law model. The stress relaxation studies also supported that both materials were physical networks. The linear and non-linear viscoelastic behaviours for both water-starch-PAAm and DMSO-starch-PAAm copolymer graft copolymer gels were similar to those of some cosmetic and wound healing gels, which suggested that these modified starch materials would be good candidates for application in cosmetic and skin wound healing products.
Acknowledgement
The technical assistance of Kelly Utt is gratefully acknowledged. This work was financially supported by US Department of Agriculture, Agricultural Research Service.
References
- Weaver MO, Bagley EB, Fanta GF, Doane WM (1974) Gel sheets produced by hydration of films from the potassium salt of hydrolyzed starch-polyacrylonitrile graft copolymer. Appl Polymer Symp 25: 97-102
- Weaver MO, Bagley EB, Fanta GF, Doane WM (1976) Method of reducing water content of emulsions, suspensions and dispersions with highly absorbent starch-containing polymeric compositions. US Patent 3,935,099.
- Fanta GF (1996) Starch graft copolymers. Salamone JC edit, Polymeric materials encyclopedia. CRC Press, Boca Raton, FL, pp: 7901-7910
- Zohuriaan Mehr MJ, Kabiri K (2008) Superabsorbent polymer materials: A review. Iran polym J 17: 451-477.
- 5. Guo WY, Peng B (2012) Synthesis and characterization of starch-graft-polyacrylamide copolymers and their application as filtration control agents in drilling fluid. J vinyl addit techn 18: 261-266.
- Guo WY, Peng B (2012) Synthesis and characterization of starch-graft-polyacrylamide copolymers and their application as filtration control agents in drilling fluid. J vinyl addit techn 18: 261-266
- Nakason C, Wohmang T, Kaesaman A, Kiatkamjornwong S (2010) Preparation of cassava starch-graft-polyacrylamide superabsorbents and associated composites by reactive blending. Carbohydr Polym 81: 348-357
- Singh AV, Nath LK, Guha M (2011) Microwave assisted synthesis and characterization of Sago starch-g-acrylamide. Starch-Starke63: 740-745
- Finkenstadt VL ,Willett JL(2005) Reactive extrusion of starch-polyacrylamide graft copolymers: Effects of monomer-starch ratio and moisture content. Macromol Chem Phys206: 1648-1652
- Willett JL ,Finkenstadt VL (2006) Reactive extrusion of starch-polyacrylamide graft copolymers using various starches. J Polym Environ 14: 125-129
- Valdebenito A, Encinas MV (2010) Effect of solvent on the free radical polymerization of N, N-dimethylacrylamide. Polym Int 59: 1246-1251
- Fernández-Garcia M, Martinez JJ, Madruga EL (1998) Solvent effects on the free-radical polymerization of methyl methacrylate. Polym39: 991-995
- Willett JL, Finkenstadt VL (2006) Initiator effects in reactive extrusion of starch-polyacrylamide graft copolymers. J Appl Polym Sci 99: 52-58
- Xu J, Bietz JA, Felker FC, Carriere CJ, Wirtz D (2001) Rheological properties of vital wheat gluten suspensions. Cereal Chem78: 181-185
- Ferry JD (1980) Viscoelastic properties of polymers. 3rd Ed. John Wiley & Sons: New York.
- Clark AH Gels and Gelling, Schwartzberg HG, Hartel RW Eds, Dekker M (1992) Physical chemistry of foods Inc. New York
- Barry BW, Meyer MC (1979) The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int J Pharm 2: 1-25
- Barry BW, Meyer MC (1979) The rheological properties of carbopol gels II. Oscillatory properties of carbopol gels. Int J Pharm2: 27-40
- Kim JY, Song JY, Lee EJ, Park SK (2003) Rheological properties and microstructures of carbopol gel network system. Colloid Polym Sci 281: 614-623.
- Fletcher J (2005) Understanding wound dressings: Alginates. Nurs Times 101: 53-54.
- Matthews KH, Stevens HNE, Auffret AD, Humphrey MJ, Eccleston GM (2006) Gamma-irradiation of lyophilised wound healing wafers. Int J Pharm313: 78-86
- Boateng JS, Matthews KH, Stevens HNE, Eccleston GM (2008) Wound healing dressings and drug delivery systems: A review. J Pharm Sci 97: 2892-2923
- Bird RB, Armstrong RC, Hassager O (1977) Dynamics of polymeric liquids, Wiley, New York, pp: 208-209.
Citation: Xu J, Selling GW (2017) A Comparison of the Viscoelastic Properties of Starch-polyacrylamide Graft Copolymers Produced in Dimethyl Sulfoxide and Water. Rheol: open access 1: 109.
Copyright: © 2017 Xu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5441
- [From(publication date): 0-2017 - Jul 15, 2025]
- Breakdown by view type
- HTML page views: 4394
- PDF downloads: 1047