A Comparative Study on Some Marine Macroalge and Their Biorefinery Approach for Sustainable Biofuel Generation
Received: 16-Nov-2018 / Accepted Date: 23-Nov-2018 / Published Date: 26-Nov-2018 DOI: 10.4172/2155-9910.1000264
Abstract
Background: The unremarkably identified renewable fuels commonly known are bioethanol and biodiesel. However, there are various types of fuels that are being globally investigated. These embrace bio-butanol, bio-jet fuel, bio-renewable diesel, bio-hydrogen, bio-toluene and various alternative organic compounds. There’s hefty chance for innovation in many areas as well as biomass handling and transportation, biomass size, biomass pretreatment, conversion of pre-treated biomass into biofuel. The bioactive potential of the 3 marine algae of common incidence in Chilika Lake of Odisha, specifically Chaetomorpha linum, Enteromorpha intestinalis and Gracilaria verrucosa for their utilization of potential biofuel production has been investigated.
Objective: The main purpose of this work was to produce biodiesel from three marine macroalgae Chaetomorpha linum, Enteromorpha intestinalis and Gracilaria verrucosa using transesterification process.
Materials and Methods: The three marine macroalgae sample were collected from Chilika Lake. The biomass dried with sunlight and converted into powder by mortar and pestle. Biodiesel was produced by the transesterification process.
Conclusion: A detailed study on the 3 marine macroalgae, their conservation, identification, maintenance in outlined substance, their growth dynamics and lipid content are undertaken to seek out their potentiality for production of biodiesel.
Keywords: Biofuel; Chilika Lake; Chaetomorpha linum; Enteromorpha intestinalis; Gracilaria verrucosa
Introduction
Today vitality for mechanical, business and private reason alongside power age and transportation is basically given by non-renewable energy source as fossil fuels (coal, gas, oil etc.). The exponential utilization of non-renewable energy sources has enormously added to the expansion in level of ozone harming substances. Moreover, the consistently expanding interest of non-renewable energy sources, their step by step expanding cost and shortening saves have constrained the researchers to concentrate over interchange fuel, which at long last finished in upon algal biofuel. Comparatively algae provide more oil than some other biofuel innovation, which is presently being utilized. Algal species can accumulate significant amounts of lipid, sometimes even half of their biomass. Algae have a place with a huge assorted gathering of straightforward, autotrophic life forms, running from unicellular to multicellular ones. The significant achievement has been accomplished on account of unicellular ones although more than one lac algal variety has been screened. The short generation time, ubiquitous presence, ability to survive under extreme condition and on nutrient depletion had made algae a promising biofuel agent.
Biotechnological exploitation of algae for human welfare is a recent phenomenon although these wonderful organisms exist on this planet since archeological era. These are responsible for changing over anoxygenic environment to oxygenic one. Various forms of algae have high biotechnological potentials and being utilized as source of novel drugs biofuel (bio-diesel, bio-gas, bio-ethanol and bio-butanol) biofertilizer, bio-pigments and dyes, food and feed, sink for greenhouse gases, soil amelioration and bioremediation. Algae products have mostly been used in the food, cosmetic and pharmaceutical industries. An expanding market for these products is a fact and is facing a new challenge of growing algae on a large scale. Although algae are a unique source of high-value compounds, their commercial application of algae to such process is low productivity of the culture, both in terms of biomass and product formation. Regardless of gigantic business applications and developing business sector request of algal items universally investigates on commercialization of these living beings are exceptionally constrained in India. Indian researches on algae are mostly limited to taxonomical, physiological, biochemical and ecological one and on the top limited too few taxa instead of algae. There is a need to identify, understand, popularize and develop algal biotechnological processes leading to establishment of algal based industries and algal based industries and algal entrepreneurship.
Shay [1] reported that algae were one of the best sources of biodiesel. In fact, algae are the highest yielding feedstock for biodiesel. It can produce up to 250 times the amount of oil per acre as soybeans. Producing biodiesel from algae might be just the best approach to create enough automobile fuel to supplant current gas utilization. Algae produce 7-31 times greater oil than palm oil. It is very simple to extract oil from algae.
Macroalgae can be defined as a diverse and distantly related nonphylogenetic group of macroscopic aquatic eukaryotes belonging to Chlorophyta (Green algae), Rhodophyta (red algae), Phaeophyceae (brown algae) and Xanthophyceae (yellow-green algae) [2,3]. In a sustainable production of biofuels, macroalgae have several potential advantages compared to terrestrial plants [4]. Macroalgae do not need agricultural land or fresh water to grow [5]. The chemical compounds in macroalgae can be used in several applications in food, fodder or chemical industry.
Algal culture systems
Culture frameworks are altogether different between macroalgae (seaweed) and microalgae. Considering their little (μm) measure, microalgae can be cultured in a framework intended for that reason while seaweed can be developed specifically in the untamed ocean. The primary notice of seaweed culture goes back to 1690, in Japan [6].
Algal products
Complete cell biomass: Drying requires a lot of vitality, which has a solid negative impact on the vitality equalization and capital expenses of required gear [7]. Thermochemical liquefaction is a hightemperature, high-weight treatment in which a wet biomass stream can be connected, [8-10] but his innovation is still a work in progress and is probably going to require no less than five years previously it tends to be financially connected [11]. There is 55-75 percent methane in biogas which can be combusted to create warm as well as power or moved up to supplant flammable gas [12].
Carbohydrates and ethanol: With new advances, cellulose and hemicellulose can be hydrolyzed to sugars making the likelihood of changing over a much bigger piece of algal dry issue to ethanol [13]. Another green growth innovation for ethanol generation is being created, in which green growth are hereditarily adjusted to deliver ethanol from daylight and carbon dioxide [14].
Hydrocarbon: One algal species Botryococcus braunii is well known for its ability to produce hydrocarbons which have been loosely described as equivalent to the “gas-oil fraction of crude oil” [15]. These hydrocarbons can be transformed into fuel, lamp oil and diesel like petroleum.
Hydrogen: As a vitality transporter, hydrogen offers extraordinary guarantee as a fuel since it tends to be connected in versatile applications with just water as fumes and no outflows when utilized in a power device. At present, hydrogen gas is created by the procedure of steam transformation of petroleum derivatives. Extensive scale electrolysis of water is likewise conceivable, yet this creation strategy costs more power than can be produced from the hydrogen it yields.
Material and Methods
Description of the experimental site
Context: To enable six-month dissertation Programme of M. Sc (Biotechnology) of Ravenshaw University, Cuttack, Odisha, The Centre for Environmental Studies on 19th February 2017, constituted a team to visit Chilika Lake in Odisha, for collecting samples like macro algae.
Visit itinerary: The team comprising of twelve members is Dr. Rajesh Kumar Mohanta, DST Project Coordinator, CES, Odisha, Chita Ranjan Sahoo, Ph.D. (Research Scholar), eight M.Sc. students from Berhempur University and two M.Sc. students from Ravenshaw University.
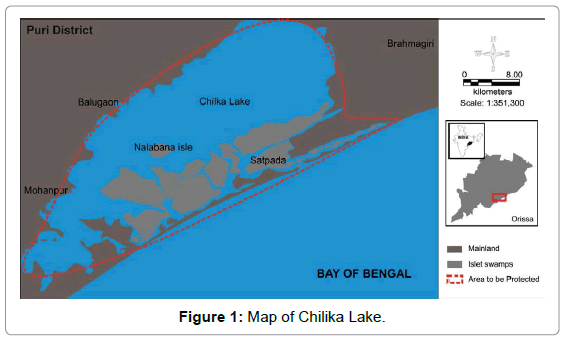
The Chilika lake: It is the largest brackish water lagoon in Asia with estuarine character and the largest wintering ground for migratory water-fowls found on the Indian sub-continent. The area of the lagoon varies between 1165 and 906 sq. km during the monsoon and summer, respectively (Figure 1).
Field visit: On 19th February 2017, our team visited Balugaon and Kalijai islands. We collected various algae samples mainly red and green algae (seaweeds) in and around kalijai area (Figure 2). There was also measurement of pH and salinity by pH meter and salinometer respectively at both kalijai and chilika. The samples were deposited at CES, Bhubaneswar for further processing.
Collection of algae samples
Algae sample were collected from a depth of 10-15 cm. from the surface of water by hands. Phytoplankton were concentrated by filtration of water sample through a membrane filter (i.e. millepore) from which they are washed and examined under microscope. Concentration could be achieved by centrifuge with clinical centrifuge. Algae could be concentrated by adding preservatives to water samples and permitting the material to settle, to the bottom of the vessel. Some surface members of phytoplankton were collected with the help of mesh also. The time of sampling ranged between 8am-11am throughout the study period. For surface sample quantification, 50 liters of surface was passed through plankton net & collected samples were transferred to plastic bottles & were returned to laboratory. It was washed several times in tap water followed by distilled water in Petri dishes and observed under a dissection of microscope and immediately fixed by staining with aniline blue and glycerin for identification purposes.
Distilled water was used to for preparation of all solutions & media used in the experiment. Stock solution of each of the chemicals were prepared to 100ml volume in the double distilled water. The salt solution was autoclaved at 15lb square inch pressure for 30 min & were stored at room temperature for use. To avoid precipitation, phosphate was added to sterile media, after cooling under aseptic conditions (Table 1).
| CHMICALS | mg/L |
|---|---|
| 1. MnCl2 | 0.1 |
| 2. H3BO3 | 0.1 |
| 3.NA2MoO4.2H2O | 0.1 |
| 4. CuSO4.5H2O | 0.1 |
| 5. ZnSO4.7H2O | 0.1 |
Table 1: Micronutrient solutions.
Brief note on the test organism
Macroalgae are classified as Phaeophyta or brown algae, Rhodophyta or red algae and Chlorophyta or green algae based on composition of photosynthetic pigments. The green macroalgae have evolutionary and biochemical affinity with higher plants.
Enteromorpha intestinalis: It belongs to the Phylum-Chlorophyta, Class-Chlorophyceae and Order-Ulvales (Figure 3). It is a green seaweed, comprising of swelled, rounded fronds that develop from a little discoid base. Fronds are regularly unbranched, they might be 10- 30 centimeters in length or much more and 6-18 millimeters in distance across, generally with rounded tips. It Grows profusely in rocks. It is an annual plant, framing masses of dyed white fronds towards the end of the season. It might grow up to 1 meter in tallness, at the speed of 0.15-0.25 centimeters every day. It is euryhaline: it endures wide ranges in the salty water.
Chaetomorpha linum: It belongs to the phylum-Chlorophyta, Class-Ulvophyceae and Order-Cladophorales (Figure 4). Chaetomorpha linum is a seaweed with fine hair-like, uniseriate, unbranched fibers. The plant is found with free-gliding in the lake in masses, appended on rocks, shells and different items and as free drifting. Plants are yellowish green in color, 100-375 μm diameter, 1-2 times as long as broad. The unattached plants frame masses of curved filaments. The appended fiber develops as tufts from an unmistakable base.
Gracilaria verrucose: It belongs to the Phylum-Rhdophyta, Class-Florideophyceae and Order-Gracilariales (Figure 5). It Grows abundantly on rocks, boulders and another harder substratum. Plants are 1 m long and profoundly stretched. The plant gets dyed as it gets presented to sunlight.
Artificial sea water
Artificial seawater (ASW) is a mixture of dissolved mineral salts that stimulates seawater. It is primarily used in marine biology, marine and reef aquaria. It allows the easy preparation of media appropriate for marine organisms such as algae, bacteria, plants and animals. Artificial seawater has the advantage of reproducibility over natural seawater (Table 2).
| REAGENT | QUANTITY FOR 1 LIT | FINAL CONC. |
|---|---|---|
| NaCl | 26.29 g | 450 mm |
| KCl | 0.74 g | 10 mm |
| CaCl2 | 0.99 g | 9 mm |
| MgCl2 (6H2O) | 6.09 g | 30 mm |
| MgSO4 (7H2O) | 3.94 g | 16 mm |
Table 2: Composition of artificial sea water.
Artificial marine medium
Marine artificial media are used to minimize or exclude known contaminants for the purpose of studying trace elements. The artificial sea water recipe consists of mineral salts, some anhydrous salts that can be weighed out, and some hydrous salts that should be added to the artificial seawater as a solution. All the salts present in the medium provides organic source of growth nutrients. One of such media is Pravasoli medium (Table 3a and 3b).
| P II + Trace metals | per litre (1000 ml) | |
| Na2EDTA | 1.0 g | |
| H3BO3(Boric Acid) | 1.12 g | |
| MnSO4.7H2O | 0.12 g | |
| ZnSO4. 7H2O | 0.022 g | |
| CuSO4. 7H2O | g | |
| Iron-EDTA | Fe(NH4)2(SO4)2. 6H2O | 0.7 g |
| Na2 EDTA | 0.6g |
Table 3a: Composition of artificial marine medium (Stock Solution).
| Component | STOCK | PER LITRE |
|---|---|---|
| Na2β. Glycol PO4. 5H2O | 50 g /1 lit | 8.0 ml |
| NaNO3 | 35 g /1 lit | 110.0 |
| Iron- EDTA (2) | 100.0ml | |
| Vitamin B12 | 0.01 g / 1 lit | 8.75 ml |
| Thiamine | 0.5 g / 1 lit | 8.0 ml |
| Biotin | 0.005 g / 1 lit | 200 ml |
| P II (1) + Trace metal | 200 ml |
Table 3b: Composition of Pravasoli Medium.
Drying of biomass
The drying process is one of the major limitations in the production of low-cost commodities (fuel, food, feed) and high value products (β-carotene, polysaccharides). The process to be selected depends on the final product desired. The use of dehydration increases the shelf life of the biomass as well as final product.
Several methods have been used to dry macroalgae. Some of the most widely used include spray drying, drum drying, freeze drying and sun drying [16]. In this research work, sun-drying technique has been utilized. Because of the high water-content, the drying procedure took a few days for transform muggy biomass into water-free green algae biomass. Then the dry biomass has been changed over into powdered mass using mortar and pestle. The spray-drying method is not economically feasible for low-value products such as biofuels and protein [17].
Oil extraction
Debris was separated from the three marine macroalgae Chaetomorpha linum, Enteromorpha intestinalis and Gracilaria verrucosa followed by washing with running water and distilled water. 50 g of wet weight from each sample was taken. Paste of all the three samples was prepared by using mortar and pestle and then dried in the incubator at 80°C for 30 minutes. 50 g of wet weight became 12 g, 10 g and 5 g dry weight respectively. Then the dry algae biomass was blended with chloroform and methanol and shook vigorously for 10 minutes. Then NaCl solution added in the mixture and shook for another 10 min. Resulted mixture was filtered to remove the algal biomass. The clear solution kept for a while to form two distinct phases. The upper phase which contains water and methanol are separated from the lower chloroform-oil phase and discarded. The lower chloroform-oil phase was evaporated to remove excess chloroform and the remaining solution was used for the further treatment. The process was repeated for all the three samples.
Biodiesel production
Alkaline catalyst sodium hydroxide (NaOH) was dissolved in the methanol (CH3OH) by hand shaking and whirling. Resulting sodium methoxide (CH3NaO) was then added to the preheated (at 50°C) algal oil and air tight the reaction system to avoid the loss of methanol. Temperature was kept constant at 55-65°C and heated for 3h to complete the reaction. Once the reaction was completed, two major products exist- biodiesel and glycerin (C3H8O3). Biodiesel was separated from glycerin by gravity settling as the glycerin was much denser than biodiesel, it settled down at the bottom.
Separated biodiesel contained some soap and methanol. The methanol was removed by vaporization. After the methanol had been removed, the biodiesel was washed with distilled water by liquidliquid extraction process to remove the soap and catalyst. The washing procedure was repeated for 3-4 times until the soap totally removed. Remaining water present in the biodiesel was removed by heating it at 100°C for 10 min. Finally, usable form of biodiesel was found.
Transesterification process
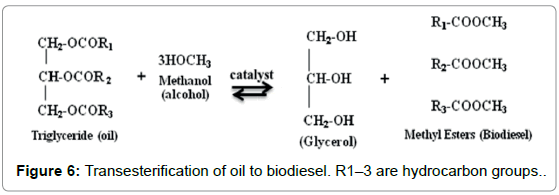
This is a procedure to change over algal oil to biodiesel, which includes numerous steps of reactions between triglycerides or unsaturated fatty acids and alcohol. Various types of alcohols, for example, ethanol, butanol, methanol, propanol, and amyl alcohol can be utilized for this reaction. However, ethanol and methanol are frequently used for the commercial improvement because of its minimal effort and its physical and concoction preferences [18,19]. In this technique, around 3 mol of alcohol are required for every mole of triglyceride to form 3 mol of methyl esters (biodiesel) and 1 mol of glycerol (result) (Figure 6) [19-23]. Glycerol is denser than biodiesel and can be intermittently or consistently expelled from the reactor with the end goal to drive the equilibrium reaction. In this manner, the biodiesel is recouped by continued washing with water to expel glycerol and methanol [21].
Result and Discussion
Biochemical composition (biomass, lipid content and percentage of oil) in the experimental algae
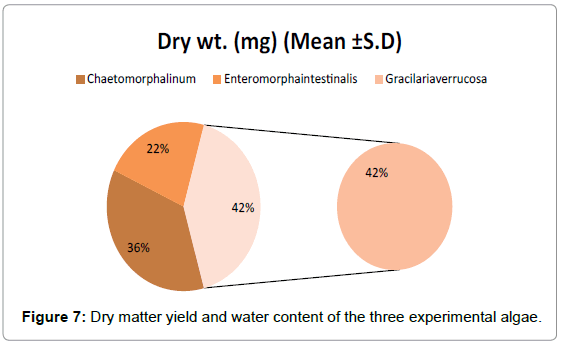
Dry wt. and water content (Table 4 and Figure 7): Fresh wt. and dry wt. estimation were made from the pooled samples of the algae. The dry matter yield for Chaetomorpha linum was 180 mg/dry wt. and water content 820 mg. For Enteromorpha intestinalis was 110mg/dry wt. and water content 890 mg. and 205 mg/dry wt. for Gracilaria verrucosa while water content 795 mg. indicating the high-water content of the seaweed Enteromorpha intestinalis.
| Algae | Dry wt. (mg) | Water content |
|---|---|---|
| Chaetomorphalinum | 220 | 780 |
| Enteromorphaintestinalis | 110 | 890 |
| Gracilariaverrucosa | 205 | 795 |
Table 4: Represents the dry matter yield and water content of the three experimental algae. Dry content of G. verrucosa has the high value of dry content i.e. 205 mg/dry wt as compared to Enteromorpha intestinalis (110) and C. linum (180). Dry matter was taken from 1 kg of total biomass. Water content was highest in E. intestinalis (890) as compared to C. linum and G. verrucosa.
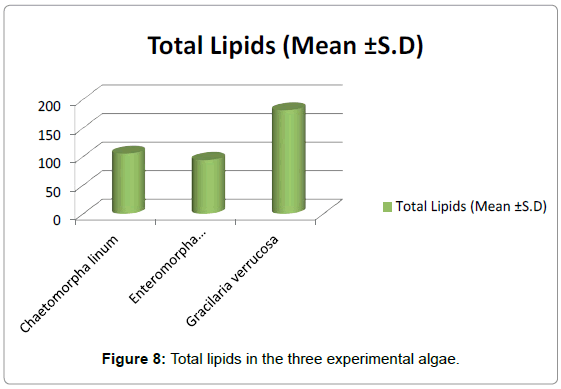
Lipids content of the three experimental algae (Table 5 and Figure 8): The level of lipids was quite high in the dried powdered matter of Gracilaria verrucosa as compared to Chaetomorpha linum and Enteromorpha intestinalis. In Chaetomorpha, the lipid content was 2.308 mg/g dry wt. In Enteromorpha, the lipid content was 1.856 mg/g dry wt. while in Gracilaria, the lipid content was 1.155 mg/g dry wt.
| Algae | Total Lipids (Mean ±S.F) |
|---|---|
| Chaetomorpha linum | 105 |
| Enteromorpha intestinalis | 94 |
| Gracilaria verrucosa | 181 |
Table 5: Represents total lipid contents of three experimental algae, lipid content was highest in G. verrucosa (181). E. intestinalis has lipid content (94) and C. linum has lipid content (105).
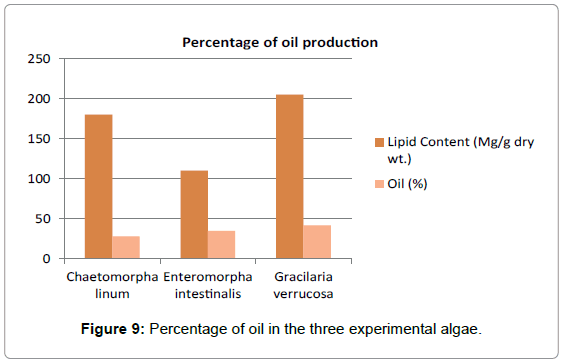
Oil content (Table 6 and Figure 9): The oil content was quite high in the dried powdered material of Gracilaria verrucosa amounted to be 42% dry wt. (Figure 10). The result shows that the three experimental organisms of Chilika Lake can be used for the sustainable development of the environment due to its abundance in Chilika Lake. The high lipid and oil percentage has shown its further biotechnological utilization for biofuel production.
| Algae | Biomass (Mg) | Lipid Content (Mg/g dry wt.) |
Oil (%) |
|---|---|---|---|
| Chaetomorpha linum | 1000 | 180 | 28 |
| Enteromorpha intestinalis | 1000 | 110 | 35 |
| Gracilaria verrucosa | 1000 | 205 | 42 |
Table 6: Shows the percentage of oil content three experimental algae. Oil content of G. verrucosa was more than C. linum and E. intestinalis.
Conclusion
A detailed study on their conservation, identification, maintenance in defined culture medium, their growth dynamics and lipid content have been undertaken to find out their potentiality for production of biodiesel (oil extraction from algae) and highest lipid content Gracilaria verrucosa>Enteromorpha intestinalis>Chaetomorpha linum respectively.
Biofuel from macroalgae, will be better choices as far as fuel necessities for quickest developing economies such as India. Government advances little scale enterprises for financial improvement and business age, yet economy is in the interim developing with numerous ecological and social issues in such economies. From the author’s point of view, the coming years or decade will observe the tangle development of macroalgae advances. With the handling innovation and major research going close by, we can expect this new stream of normal asset truly being tapped.
References
- Shay EG (1993) Diesel fuel from vegetable oils: Status and opportunities. Biomass Bioenergy. 4: 227-242.
- Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am. J. Bot 91: 1535-56.
- Graham LE, Wilcox LW (2009) Algae, 2ndedn. Benjamin Cummings, San Francisco, CA.
- John RP, Anisha GS (2011) Macroalgae and their potential for biofuel. CAB Reviews. 6: 1-15.
- Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J. App. Phycol 6: 45-60.
- Buck BH, Buchholz CM (2004) "The offshore-ring: A new system design for the open ocean aquaculture of macroalgae." Journal of Applied Phycology. 16: 355-368.
- Banerjee A, Sharma R, Chisti Y, Banerjee UC (2002) "Botryococcus braunii: A renewable source of hydrocarbons and other chemicals." Crit. Rev. Biotechnol 22: 245-279.
- Dote Y, Sawayama S, Inoue S, Minowa T, Yokoyama S et al. (1994) "Recovery of Liquid Fuel from Hydrocarbon-Rich Microalgae by Thermochemical Liquefaction." Fuel. 73: 1855-1857.
- Tsukahara K, Sawayama S (2005) "Liquid fuel production using microalgae." J. Jpn. Pet. Inst 48: 251-259.
- Meuleman B (2007) Personal communication on biomass conversion technology.
- Mes TZD, Stams AJM, Reith JH, Zeeman G (2003) Methane production by anaerobic digestion of wastewater and solid wastes. Biomethane & bio-hydrogen: status and perspectives of biological methane and hydrogen production. 58-102.
- Hamelinck CN, van Hooijdonk G, Faaij APC (2005) "Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term." Biomass Bioenerg 28: 384-410.
- Deng MD, Coleman JR (1999) "Ethanol synthesis by genetic engineering in cyanobacteria." Appl. Environ. Microbiol 65: 523-528.
- Hillen LW, Pollard G, Wake LV, White N (1982) "Hydrocracking of The Oils of Botryococcus-Braunii To Transport Fuels." Biotechnol. Bioeng 24: 193-205.
- Richmond A (2004) Handbook of microalgal culture: biotechnology and applied phycology. Pondicherry, Tamil Nadu, India: Blackwell Science Ltd.
- Mata TM, Martins AA (2010) Caetano NS Microalgae for biodiesel production and other applications: A review. Ren Sustain Energy Rev 14: 217-232.
- Bisen PS, Sanodiya BS, Thakur GS, Baghel RK, Prasad GBKS (2010) Biodiesel production with special emphasis on lipase-catalyzed transesterification. Biotechnol. Lett 32: 1019-1030.
- Susilaningsih D, Djohan AC, Widyaningrum DN, Anam K (2009) Biodiesel from indigenous Indonesian marine microalgae Nanochloropsis sp. J. Biotechnol. Res. Trop. Reg 2: 1-4.
- Meher LC, Vidya SD, Naik SN (2006) Technical aspects of biodiesel production by transesterification – a review. Renew. Sustain. Energ. Rev 10: 248-268.
- Sharma YC, Singh B (2009) Development of biodiesel: current scenario. Renew. Sustain Energ Rev 13: 1646-1651.
- Stergiou PY, Foukis A, Filippou M, Koukouritaki M, Parapouli M et al. (2013) Advances in lipase-catalyzed esterification reactions. Biotechnol Adv 31: 1846–1859.
Citation: Mohapatra S, Padhi S (2018) A Comparative Study on Some Marine Macroalge and Their Biorefinery Approach for Sustainable Biofuel Generation. J Marine Sci Res Dev 8: 264. DOI: 10.4172/2155-9910.1000264
Copyright: © 2018 Mohapatra S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4516
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3572
- PDF downloads: 944