A Comparative Study of the Fixative Properties of Honey and Formalin
Received: 23-Dec-2022 / Manuscript No. DPO-22-84515 / Editor assigned: 26-Dec-2022 / PreQC No. DPO-22-84515 / Reviewed: 09-Jan-2023 / QC No. DPO-22-84515 / Revised: 16-Jan-2023 / Manuscript No. DPO-22-84515 / Accepted Date: 16-Jan-2023 / Published Date: 23-Jan-2023 DOI: 10.4172/2476-2024.1000212
Abstract
Before a routine histological or cytological examination, tissues are fixed, maintained, and guarded against putrefaction and autolysis. The fixative is most frequently used to fix tissues in formalin. Because of its toxicity using formalin poses health risks, according to the Occupational Safety and Health Administration (OSHA) we want to use a natural alternative such as honey in a formalin-free laboratory for the preservation of clinical specimens. Honey is a naturally occurring substance with antibacterial, acidic and dehydrating qualities that make it a fixative. This study compares the effectiveness of formalin and honey as fixative agents. A rat liver tissue sample, formalin, honey, alcohol, xylene, cassette, mold, paraffin wax, rotary microtome, floating bath, slides, rack, and hematoxylin and eosin for staining are all required for this study. A liver tissue biopsy will be fixed with formalin and honey respectively for 24 hours while being grossed and recorded every four hours. It will go through dehydration, clearing, grossing, paraffin wax, hematoxylin, eosin staining and microscopic examination. The anticipated outcomes will demonstrate statistically significant changes in nuclear specificity and cytoplasmic staining between honey and formalin samples. A a substitute for formalin and as a tissue fixative honey is readily available has no known toxicity, and can be employed. However research should be done to identify ways to get rid of the drawbacks, including homogenization, seen with connective tissue.
Keywords: Formalin; Fixation; Honey; Tissue fixative; Tissue processing; H and E staining
Introduction
Background
Fixation is the first and most crucial step in preparing tissues for microscopic examination. Fixation preserves tissues to maintain the integrity of the tissue and to withstand subsequent treatment with other reagents without loss, distortion, or decomposition. It also stops autolysis and putrefaction and maintains tissues in a state that allows for proper staining [1]. Fixation is also used to contrast the tissues in a particular area that are normal and pathological. There are several fixatives used for tissue fixation, including glutaraldehyde, alcohol and others, but formaldehyde is the one most frequently employed in diagnostic histopathology, according to Bancroft JD, et al. Treatment of the tissue as soon as it is taken out of the body is necessary for any effective microscopic preparation. Histopathological methods are built on this phenomenon. The tissue needs to be moved and repaired right away in the proper fixative solution [2].
After death when a tissue or organ is removed from the body autolysis and putrefaction takes place [3]. This is because the organ or tissue is destroyed by intracellular enzymes found in the lysosomes, autolysis is more similar to apoptosis than necrosis, while putrefaction is similar to necrosis because bacteria present in the tissue cause it to decay [4]. To prevent cell death and ensure correct tissue preparation and processing for microscopic analysis, the tissue must thus be preserved.
Neutral buffered formalin a 10 % solution of formalin buffered at pH 7.2-7.4 is used in almost all laboratories throughout the world. Due to its low cost and simplicity of availability, formalin is frequently used as a standard examination for tissue fixing and preservation.
Formalin on the other hand puts human health and safety at serious risk. It became known that formaldehyde may cause cancer after the US passed the Formaldehyde Standard (29 CFR 1910.1048 Formaldehyde) rule in 1987 [4]. This is according to the agency for toxic substances and disease registry, formalin causes cancer, irritation of the skin, eyes, respiratory, digestive systems, neurotoxicity, reproductive abnormalities and developmental problems.
By substituting less dangerous compounds for formaldehyde efforts have been made to identify non-toxic safer replacements. Honey has long been demonstrated to possess antimicrobial qualities and the ability to preserve compounds without having any negative impact on its users. Meat was preserved for several days in ancient Rome using honey [5-7].
Honey, a natural and safe fluid, produced by the stinging bee Apis mellifera, is employed as a fixative [3]. The antibacterial, anti-autolytic, antimicrobial, antioxidant and tissue hardening characteristics of honey contribute to its capacity to repair tissues. Honey is known to retain tissue morphology in comparison to that created by formalin, according to additional honey has astringent, drying, and antimicrobial effects. Several substances found in honey aid in tissue damage prevention. Natural honey has a pH of about 4.0 due to the presence of amino and organic acids.
Since honey has an acidic pH of 4.0, the majority of microorganisms will be killed by it and the sterile environment creates allows the honey and tissues to last for a very long time. Organic acids like gluconic acid, acetic acid, lactic acid, citric acid, formic acid and fumaric acid are the source of the acidic pH. Therefore it will be a good substitute for formalin, according to Saore. Previous studies have demonstrated that tissues preserve in low honey concentrations and stained with hematoxylin and eosin produced outcomes that were superior to those produced by tissues fixed in formalin as the control. The duration of fixation and the impacts on tissues and the staining reaction of honey and formalin are all compared in this study to show how well they may be used for histological fixation.
Materials and Methods
Study design
This is an observation and experimental study. Rats were obtained from Kwame Nkrumah University of Science and Technology in Kumasi, Ashanti region- Ghana.
Sampling technique
The dissection of the rats and fixing of the tissues in their corresponding concentrations was also done at KNUST animal husbandry. The samples were then transported to Hope Xchange medical center for further processing of the tissue to be done. The honey used was acquired locally from an apiculturist at Damango in the Northern Region with all other necessary reagents, materials and apparatus from Hope Xchange Medical Center.
Purposive sampling
The specimen for the study were fresh tissue samples namely; heart, liver, spleen and kidney harvested from the rats and fixed in serial concentration of honey and 10% NBF as control.
Study site
The research work was conducted at histopathology laboratory department of Hope Xchange Medical center located in Santasi, Ashanti region-Ghana. The facility doubles as a research center and also a catalyst in addressing rampant diseases and ailments with a G.P.S address of AK-W362-2858.
Materials/Reagents
Formalin, aqueous solution (distilled water), transparent containers, fresh honey, dissection kits, chloroform, measuring cylinder, cotton wool, conical flask, pH meter (Extech® Instrument), automated staining machine (Tissue Tek Prisma Plus), microtome (Accu-Cut SRM), paraffin embedding station (Tissue Tek TEC), slides, thermometer (Checktemp®), Refractometer (Matronix Q30®), colorimeter (NEXTTEQ®). The other reagents and materials used were alcohol as a dehydrant, xylene as a clearing agent, cassette, mold, paraffin wax, floating bath, slides, rack, hematoxylin and eosin for staining. The honey used was acquired locally from an apiculturist at Damango in the Northern Region, Ghana with all other necessary reagents, materials and apparatus acquired from Hope Xchange Medical Center.
Fixative preparation methods
• 1 part of the formalin is added to 9 parts of distilled water.
• To achieve the 300 ml of the working solution, add 30mls of the formalin into 270 ml of distilled water.
• The formalin was poured into a transparent container labelled 10% neutral buffered formalin modified [8].
Preparation of honey
• The honey was prepared in 25%, 50%, 75%, 100% concentrations.
• At 25% concentration, 75 ml of the honey is mixed with 225 ml of distilled water uniformly.
• At 50% concentration, 150 ml of the honey is added to 150 ml of the distilled water and stirred.
• At 75% concentration, 225 ml of the honey was added to 75 ml of the distilled water to form a uniform mixture.
• At 100% concentration, an undiluted 300 ml of honey was used.
• Each of the prepared concentrations was poured in their well labelled respective containers modified (Figure 1).
Physicochemical testing for honey
The physicochemical properties of the honey were tested to determine the glucose content, color, temperature and pH of the honey [8].
• A colorimeter (NEXTTEQ®) was used to measure the absorbance of honey at 540 nm and a pH meter (Extech® Instrument) was used to determine the honey's pH [3].
• The glucose content was measured using a refractometer (Matronix Q30®) by glucose-oxidase colorimetric technology, which is based on the glucose oxidase-peroxidaseaminophenazone principle. The glucose concentration was estimated in mmol/L [9].
• The temperature of honey was measured with a thermometer (Checktemp®). It was measured in degree celsius [10].
Extraction of organs and processing
The test subjects for the study were rats. Five rats were used and four organs namely the liver, heart, spleen and kidney. Tissue samples were harvested at the KNUST animal husbandry by sedating the rat with chloroform before excision. The dissection of the rats was done to excise the liver, heart, spleen and kidney. The harvested tissues were washed in 10% concentration of honey and 10% Neutral Buffered Formalin (NBF) for the control.
The washing was done because the fresh tissues were bloody, so washing them in the various concentrations of the honey and formalin before fixing the tissues enhanced the isotonicity of the tissues to the fixatives modified (Figure 2) [8].
Protocol for tissue extraction and fixation
Before the dissection, the rats were sedated with 96% concentration, chloroform (CHCl3) for 30 mins. Cotton was soaked with the chloroform and was placed in their fume hood.
• After 30 minutes the rats were sedated, the dissection kits were ready for the incisions to be made.
• The unconscious rat was placed on their ventral side and pinned unto a tray letting their abdomen faced upwards.
• The tray was disinfected with 70% ethanol.
• A surgical blade was used to peel off the fur around the lower abdomen.
• Forceps was used to pinch and slightly pull the skin upwards.
• An incision was made through the abdominal cavity with a surgical scissors starting from the lower abdominal cavity through the midline and ending at the chain.
• After removing the skin, the epidermis, muscles which has the dermis was cut to expose internal organs.
• Using the forceps one side of the skin was grabbed at one side and another incision was made diagonally towards the back paw with a surgical scissors.
• The same step was done at the opposite side.
• To locate the heart, the ribcage was cut to expose and remove the pericardium around the heart and excised.
• The liver, spleen, right and left kidney were all excised.
• Grossing was done by measuring the length, width, and height of the organs for the size.
Heart was 1.6 by 1 by 0.5,
Liver was 3.8 by 4.8 by 0,
Kidney was 1.3 by 0.8 by 0.3,
Spleen was 3.8 by 0.7 by 0.4
• The consistency was smooth, soft, fragile and bloody
• The liver, spleen, heart and the kidney that were to be fixed in the formalin which is our control were washed in 10% NBF to remove excess blood.
• The liver, spleen, heart and the kidney to be fixed in honey were similarly washed in 10% concentration of the honey before fixing in their various serial concentration (25%,50%,75%,100%)
• Each concentration of the honey contained all of the four organs with their corresponding time of fixation written on the containers.
• The fixation was done for 24 hours. At an interval of 4 hours the organs were removed for grossing to be done till the 24th hour of complete fixation.
• After 24 hours, sections of the liver and kidney for each of the concentrations of the honey (25%,50%, 75% and 100%) and formalin (10% NBF) were selected and cut and placed in a suitable labelled cassette.
• The cassettes were labelled with case ID, block number, sample type, and date using a cassette marker and pencil.
• The organs in the cassettes were subjected to tissue processing modified.
Statistical analysis
The differences in specimen morphology were compared between the baseline measurements prior to fixation and measurements obtained at 4 hour interval each for all concentrations of immersion in the fixative. The length, height and width of the liver and kidney were measured in centimeters with a ruler to calculate for the area. The area of each of the organs was measured at a four-hour interval for 24 hours. Microsoft excel was used to generate a graphical representation of the area of the measured organs against time of fixation. A double-blinded study was conducted. Three interpreters assessed the quality of fixation of the sections independently. The various parameters, such as nuclear staining, cytoplasmic staining, cell morphology, clarity of staining, uniformity of the stain and staining intensity were used.
Tissue processing procedure
• The tissues were immersed in increasing concentrations of alcohol.
70 % alcohol…………………45 minutes
80 % alcohol…………………45 minutes
96 % alcohol…………………45 minutes
100 % absolute alcohol…………………45 minutes
100% Absolute alcohol…………………1 hour
100% Absolute alcohol…………………1 hour
• The tissues were immersed in two changes of xylene
Xylene…………………1 hour
• Infiltration of the tissues were done at four changes in molten wax
Molten paraffin wax…………………45 minutes
Molten paraffin wax…………………45 minutes
Molten paraffin wax…………………1 hour
Molten paraffin wax…………………1:30 minutes.
Embedding and microtomy
• Embedding was done at an embedding station (Figures 3,4) [11].
• After tissue processing, the tissue was removed from the cassette and placed at the bottom of a mold and filled with molten wax. These processes were done on a hot plate.
• The base of the cassette with the labelling was placed on top of the tissues in the mold and top up with paraffin wax again.
• Placed on a cool plate for the tissues sections to be embedded in the wax.
• The sections are frozen and ready to be sectioned.
• The tissue block was placed in the tissue holder of the microtome.
• Water bath was set at temperature lower than the paraffin wax to prevent melting of the paraffin wax.
• Trimming was done at 12 microns to expose the tissue.
• Sectioning was done at 5 µm and was floated out on a water bath.
• The sections were picked up with a slide and placed diagonally to drain excess water.
• The slides were then moved to the hot plate to dry.
• Slides were then labelled with case ID, block number, slide number, sample type, and date using a cassette marker and pencil. (Figure 5)
• The slides were arranged in a rack for staining [12,13].
Automated staining procedure using (Tissue-Tek Prisma®)
• Place slides containing paraffin sections in a slide holder.
• Deparaffinized sections in four changes of xylene for 3 minutes each.
• Blot excess xylene before rehydrating.
• Rehydrate sections in decrease concentrations of alcohol.
• Two changes of 100% ethanol…………………3 minutes each
95% ethanol…………………3 minutes
80% ethanol…………………3 minutes
70% ethanol…………………3 minutes
• Wash under running water for 3 minutes.
• While sections are in water, skim surface of hematoxylin with tissue paper to remove oxidation particles.
• Blot excess water from slide holder before staining with hematoxylin.
• Stain in hematoxylin for 5 minutes.
• Wash under tap water for 5 minutes to allow stain to develop.
• Dip for 30 seconds in acid alcohol to remove stain.
• Blue colour appears for 5 minutes.
• Blot excess water from slide holder.
• Dip in 70% ethanol for 3 minutes.
• Stain in eosin for 2 minutes.
• Dehydrate in 1 change in 90% ethanol for 5 minutes.
• 4 changes of 100% ethanol for 5 minutes each.
• Blot excess ethanol.
• Clear in two changes of xylene for 2 minutes.
• Coverslip and mount with Distrene Plasticine Xylene (DPX) [3].
Results
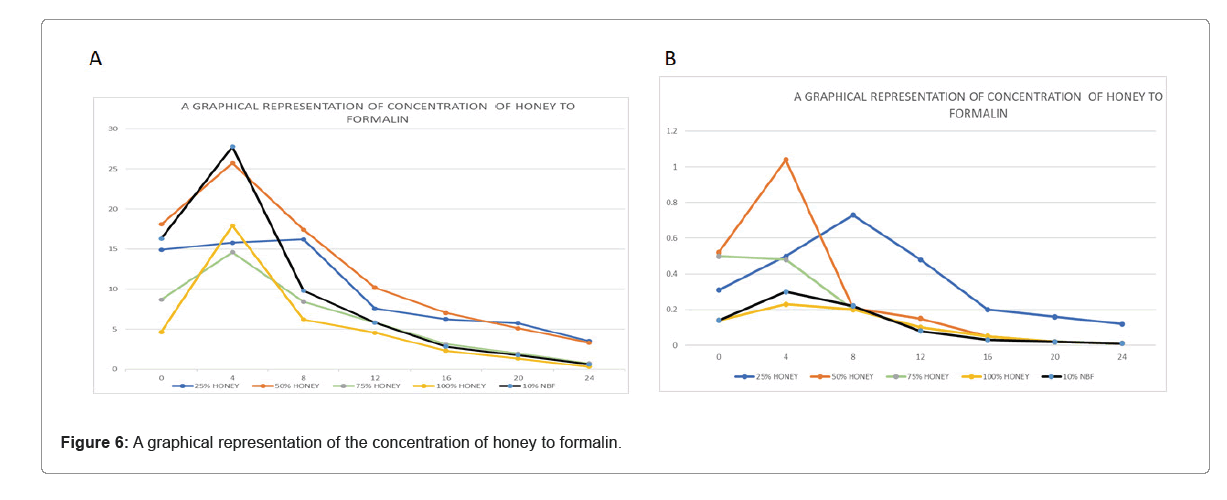
Rat organs were measured at the time of extraction in accordance with the various fixatives and concentrations. This was done to support our goal of fixing various tissues at various times and concentrations. To find out when the tissues shrunk, showing that water had been removed from a tissue to halt autolysis and putrefaction. The size of each tissue's surface in order to evaluate if the tissue has shrunk, measurements were taken every four hours. Maybe it's still sluggish. Following a day of watching the fixed tissues. Tissues fixed in 25% for both honey and formalin was seen. Compared to the other tissues in the body, honey concentration needed more time to repair the 50%, 75%, 100%, 10% NBF of honey. The fixation times for the tissues fixed in 50%, 75% and 100% were identical to those for the tissues fixed in 10% neutral buffered formalin.
In relation to the computed area of the tissues, there was an increase in the size of all the tissues at the fourth interval. Eight-hour intervals revealed shrinkage for tissue treated in 50%, 75%, 100% honey and 10% NBF. The tissues' area was drastically reduced, yet the tissues in the 25% still grew, indicating a significant shrinking. At the 12 hours to the 24 hours interval, shrinkage was consistent for all even for the tissues fix 25% concertation. of honey. The mass differences in the calculated area were due to the different sizes of the organs regardless of their fixation time (Table 1).
| Time in hours | 25% honey | 50% honey | 75% honey | 100% honey | 10% NBF |
|---|---|---|---|---|---|
| 0 | 14.9 | 18.1 | 8.68 | 4.65 | 16.3 |
| 4 | 15.8 | 25.74 | 14.6 | 17.92 | 27.8 |
| 8 | 16.2 | 17.42 | 8.4 | 6.21 | 9.8 |
| 12 | 7.57 | 10.19 | 5.83 | 4.54 | 5.8 |
| 16 | 6.24 | 7.02 | 3.12 | 2.3 | 2.8 |
| 20 | 5.74 | 5.1 | 1.94 | 1.32 | 1.73 |
| 24 | 3.5 | 3.3 | 0.72 | 0.3 | 0.6 |
Table 1: Data representing the fixation time of the various concentrations of the liver.
According to the Figure 6, the honey concentrations of the liver tissues fixed in 50%, 75% and 100% were significantly larger at the 4 hour mark than those of 25%. The size of the 25% concentration of honey increases less. For honey concentrations of 50%, 75% and 100%, shrinkage was observed after 8 hours but the 25% shrinkage did not begin for another 12 hours. Comparing them to the 10% neutral buffered formalin revealed no statistically significant differences. Despite the fact that they were all able to resolve the problems, the 75% and 100% honey had a noticeable pattern compared to the control. Although the tissues could be fixed by the 25%, it cannot be used as a long-term preservative because the tissues will disintegrate after 24 hours (Table 2).
| Time in hours | 25% honey | 50% honey | 75% honey | 100% honey | 10% NBF |
|---|---|---|---|---|---|
| 0 | 0.31 | 0.52 | 0.5 | 0.14 | 0.14 |
| 4 | 0.5 | 1.04 | 0.48 | 0.23 | 0.3 |
| 8 | 0.73 | 0.21 | 0.2 | 0.2 | 0.22 |
| 12 | 0.48 | 0.15 | 0.1 | 0.1 | 0.08 |
| 16 | 0.2 | 0.05 | 0.05 | 0.05 | 0.03 |
| 20 | 0.16 | 0.02 | 0.02 | 0.02 | 0.02 |
| 24 | 0.12 | 0.01 | 0.01 | 0.01 | 0.01 |
Table 2: Data representing the fixation time of the various concentrations of the kidney.
Comparatively to the area calculated for the liver, the size of the kidney was very small and had an effect on the calculated area in their various fixatives. The 10% NBF and the 50%, 75% and 100% did not differ statistically. At the 8-hour mark, the 10% NBF and the 50%, 75% and 100% conc. of honey all shrunk. At the 12 hour mark, honey shrinkage of 25% was observed. This demonstrates that honey can be used as a formalin substitute for both short-term and long-term fixations.
The physicochemical composition of honey was examined to enable appropriate tissue fixation. Like honey, formaldehyde includes glucose that aids in tissue fixation. Two potential processes for honey fixation have been identified in the literature. The first method involves turning carbohydrates into gluconic acid, which has a wide range of uses as a preservative in the food and pharmaceutical industries by halting the breakdown of the food. The alternative method involves the fructose in honey which at low pH levels decomposes to produce aldehyde groups. These aldehyde groups then combine with lysine amino acids to generate methylene bridges, which result in the fixing of the tissue. The effect of formaldehyde is comparable to the mechanism of honey fixation.
Physiochemical properties
The honey used in the study was good with a deep amber color and a pH 4.0 at temperature of 27ºC and glucose content was 284 mmol/L. To guarantee appropriate digestion of the tissue, each fixative was grossed for 4 hours. These tests determined if the tissues had been repaired (Table 3).
| Color | pH | Temperature | Glucose content |
|---|---|---|---|
| Deep amber | 4 | 27ºC | 284 mmol/l |
Table 3: shows the results of physicochemical properties of honey.
Rigidity
At the 4 hour mark, all of the fixatives tissues were still soft because water had entered the tissues, which caused a growth in their size. At an 8 hour interval, tissues preserved in 75%, 100% honey and 10% NBF were stiff with significant shrinkage. At the 12 hour mark, the tissues were stiff from the 25% and 50% honey.
Coloration of the tissue
The organs are stained a dark amber color to facilitate correct tissue processing. The honey produced a dark coloring, whilst the formalin had a clear tissue consistency. Regardless, it followed all the correct procedures.
Disintegration
After 24 hours, the tissues that had been fixed in the 25% honey were very soft and ready to dissolve, but they were still able to undergo all necessary tissue processing and staining.
Gross fixation study
Table 4 shows the effect of different concentrations of honey and 10% buffered formalin on gross fixation. Tissues fixed in 25% honey gave poor fixation after 24 hours. While tissues fixed in 50%, 75% and 100% honey gave good preservation after 24 hours to the formalin- fixed tissues. The production of ribbons, floating on the water bath, and sectioning did not differ significantly among any of the groups. The effects of different fixatives on the tissues were identified based on histologic examination. According to nuclear morphology, there was no significant difference between formalin and honey. The nuclear and staining uniformity revealed no discernible differences between any of the groups. For tissues that were preserved in formalin and honey, the nuclear staining was great.
| Duration | 25% honey | 50% honey | 75% honey | 100% honey | 10% NBF |
|---|---|---|---|---|---|
| 4 hours | 0 | 1 | 2 | 2 | 2 |
| 8 hours | 1 | 2 | 3 | 3 | 3 |
| 12 hours | 2 | 3 | 3 | 3 | 3 |
| 16 hours | 3 | 3 | 3 | 3 | 3 |
| 20 hours | 2 | 3 | 3 | 3 | 3 |
| 24 hours | 1 | 3 | 3 | 3 | 3 |
Note: 0:No fixation (autolysis and putrefaction present), 1:Poor fixation, 2:Moderate fixation, 3:Good fixation (autolysis and putrefaction absent)
Table 4: Shows scores for effect of formalin and different honey concentrations on gross fixation.
All tissue sections preserved in various concentrations of honey and 10% NBF demonstrated 100% staining efficiency when the nuclear staining of all stained sections was evaluated. When cytoplasmic stain was evaluated, 100% and 75% of the honey had sufficient staining patterns compared to NBF. The tissue fixed in 25% and 50% honey did however, have some holes in the cytoplasmic staining and cytoplasmic details. For tissue morphology, all of the groups were adequate. The smears fixed in the various amounts of honey and the 10% neutral buffered formalin displayed comparable staining characteristics. In terms of details like cell and nuclear size, the general tissue architecture of honey fixed smears was comparable to that of 10% NBF fixed smears.
Microscopic study
The staining characteristics of tissues fixed in different concentrations of honey. The 100%, 75% 50% and 25% concentrations gave good intense and clear nuclear and cytoplasmic staining with moderate preservation of tissue morphology with total scores. The 100% and 75% gave good intense and clear nuclear and cytoplasmic staining with good preservation of tissue morphology. The differences in staining characteristics were not statistically significant when compared with the formalin-fixed tissues.
Scores and criteria for the evaluation of stained slides
Nuclear staining: 1=Acceptable: Round smooth, and clear nuclear, 0=Unacceptable: Granular membrane, disintegrated, and out of focus.
Cytoplasmic staining: 1=Acceptable: Intracytoplasmic membrane and transparent cytoplasm, 0=Unacceptable: Disintegrated cytoplasmic membrane, granular cytoplasm, and out of focus Cell.
Cell morphology: 1=preserved: Absence of folds, no overlap, and maintained nuclear to cytoplasmic ratio, 0 = unpreserved: Overlapping cells, folded and disintegrated cells.
Clarity of staining: 1= present: Crispness in staining and transparency, 0= absent: Obliterate the nucleus and cytoplasm.
Uniformity of staining: 1=present: Uniformly stained throughout the individual cell, 0=absent: Stained in different shades of color in an individual cell.
Staining intensity: 1= present: Due to dark coloration of the honey on the tissue, 0= absent: No dark coloration.
Photomicrographs
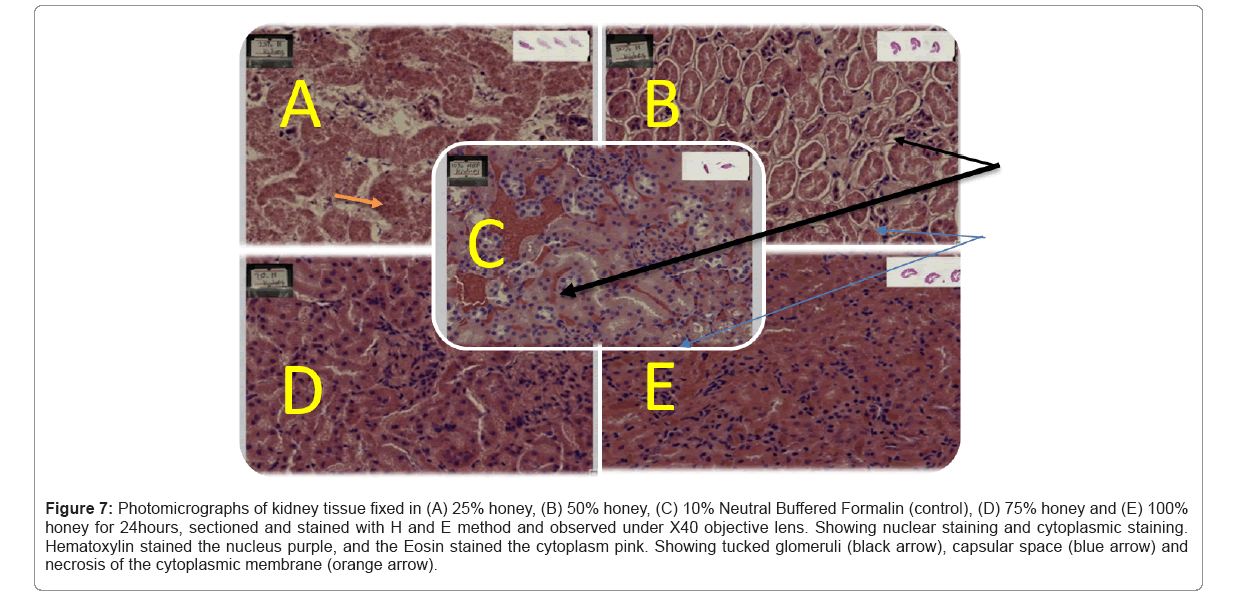
The photomicrographs are shown above Table 5 shows sections of the kidney fixed in 10% Neutral Buffered Formalin, 25% honey, 50% honey and 75% honey and 100% honey respectively and viewed at X40. Table 5 shows sections of kidney with prominent nucleus fixed in 10% NBF. Perforations of the cytoplasm was seen in 25% and 50% concentration of the kidney tissue as seen in Figures 7,8
| Features | 25% honey | 50% honey | 75% honey | 100% honey | 10% NBF |
|---|---|---|---|---|---|
| Nuclear staining | 1 | 1 | 1 | 1 | 1 |
| Cytoplasmic staining | 0 | 0 | 1 | 1 | 1 |
| Cellular morphology | 0 | 1 | 1 | 1 | 1 |
| Clarity of staining | 1 | 1 | 0 | 0 | 1 |
| Uniformity of staining | 0 | 0 | 1 | 1 | 1 |
| Intensity of the stain | 0 | 0 | 1 | 1 | 0 |
Table 5: A table showing the evaluation of the stained slides.
Figure 7: Photomicrographs of kidney tissue fixed in (A) 25% honey, (B) 50% honey, (C) 10% Neutral Buffered Formalin (control), (D) 75% honey and (E) 100% honey for 24hours, sectioned and stained with H and E method and observed under X40 objective lens. Showing nuclear staining and cytoplasmic staining. Hematoxylin stained the nucleus purple, and the Eosin stained the cytoplasm pink. Showing tucked glomeruli (black arrow), capsular space (blue arrow) and necrosis of the cytoplasmic membrane (orange arrow).
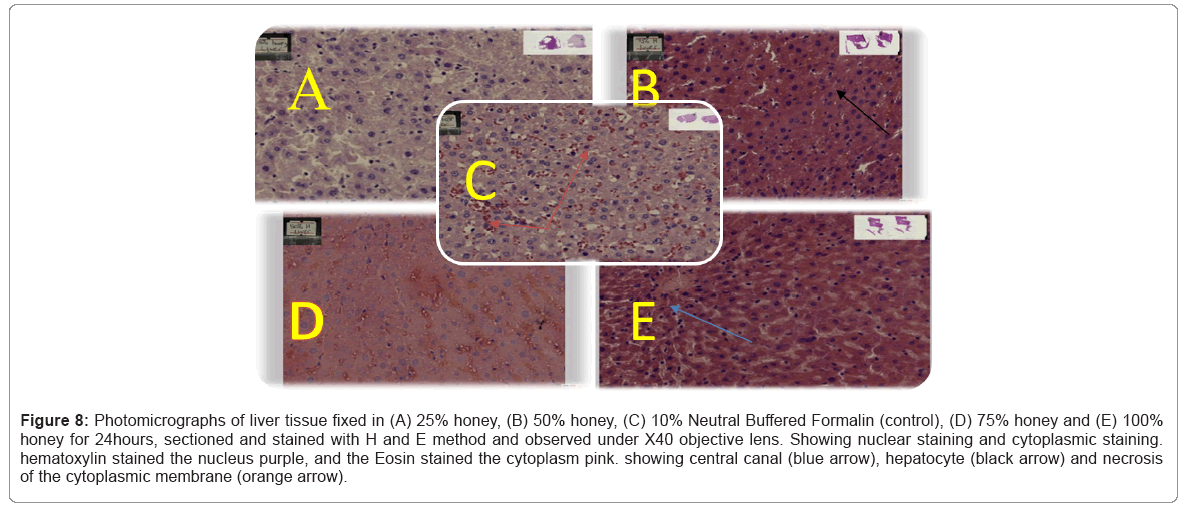
Figure 8: Photomicrographs of liver tissue fixed in (A) 25% honey, (B) 50% honey, (C) 10% Neutral Buffered Formalin (control), (D) 75% honey and (E) 100% honey for 24hours, sectioned and stained with H and E method and observed under X40 objective lens. Showing nuclear staining and cytoplasmic staining. hematoxylin stained the nucleus purple, and the Eosin stained the cytoplasm pink. showing central canal (blue arrow), hepatocyte (black arrow) and necrosis of the cytoplasmic membrane (orange arrow).
Discussion
In the present study, organs of the rats were fixed separately in formalin and different concentrations of the honey. In addition, during our study we used 25%, 50%, 75% and 100% honey fixative concentrations for 24 hours fixation time. This was to determine the duration of fixative property of honey and formalin. A four-hour interval check for shrinkage of the tissues were done for all fixatives. This was done within 24 hours fixing of the tissue. It was observed that, at the 8 hour 50%, 75% and 100% honey as well as 10% Neutral Buffered Formalin were decreased in size leading to the shrinkage of the tissues. The research concluded that 50%, 75% and 100% honey had a good preservation of tissues after 24 hours.
Tissues in the 25% concentration had a lower fixation rate because shrinkage of the tissues began at the 12 hour. Regardless of the lower fixation rate of the 25% concentration of honey, the tissues were found to be fixed after 24 hours. Moreover that of 50%, 75% and 100% honey fixation preserved tissue morphology well as compared to the 10% Neutral Buffered Formalin. The tissues were then stained with hematoxylin and eosin and were subjected to an evaluation. Hematoxylin and Eosin results showed that overall quality of tissue staining is comparable with the 10% Neutral Buffered Formalin. The nucleus was well preserved with clear nuclear details. It was observed that honey contains ascorbic acid as well as various vitamins, carbohydrates, minerals and trace elements. Low pH fixative is good for nuclear staining. In a paper by Udonkang and Inyang it was observed that the tissue morphology of fixed tissues in honey and compared with 10% neutral buffered formalin gave similar results for tissues fixed in higher concentrations of honey. It was concluded that the putrefactive nature of honey could be attributed to the presence of fatty acids, lipids, amylases and hydrogen peroxide [8].
The findings of this analysis revealed comparative results of mild differences in the staining for honey and formalin. The organs that were fixed in different concentrations of honey showed a good nuclear staining starting from the organs fixed in 100%, 75%, 50% and 25% concentration. 25 % and 50% honey concentrations showed a poor cytoplasmic staining with perforations than the 100% and 75% which gave a good cytoplasmic staining characteristic. The finding of the present study is in line with a study by Sabarinath, which reported that 10% honey, using rat liver and kidney tissues, at room temperature for 24 hour fixation gave comparable results with those obtained by formalin fixed control tissues.
A study by Özkan used the same staining criteria to evaluate honey as a substitute for formalin. They found that 10% honey is as good as 10% Neutral Buffered Formalin and suggested that honey is a safe alternative for formalin. When cytoplasmic staining was evaluated, 75% and 100% honey showed adequate staining pattern as compared with the 10% NBF. The findings of the study are in line with the present study which observed that at 50% and 75% honey, using rat liver and kidney tissues, at room temperature for 24 hour fixation gave comparable results with those obtained by formalin-fixed control tissues.
When tissue morphology was evaluated in the study 25% honey after 24 hours was ready to disintegrate and very soft but it showed adequate staining pattern when compared with 10% NBF. On analysis of clarity and uniformity of staining pattern, no significant difference was seen, suggesting that they were equivalent to NBF fixative. This shows that honey at high concentrations is suitable for museum studies and for embalmment purposes as well as histological fixation. This further justifies the use of honey for embalmment and histological fixation from a study by Özkan, et al.[10] who evaluated tissues fixed in honey for 24 hours.
The results obtained for the study further showed that for long term gross fixation and for histological staining of tissues, higher concentrations of 50%, 75% and 100% honey gave good preservation after 24 hours. This corresponds with a study by Udonkang and Inyang, where tissues were fixed in different concentrations of honey for 24 hours and subjected to routine tissue processing. The study also observed that honey is a hydroscopic (low moisture) substance because of its high sugar content. Also, the study observed that micro-organisms do not survive long in a low moisture environment. This is because water is a factor that promotes putrefaction and autolysis. This is similar with a study by Udonkang and Inyang where he observed that the fixing properties of honey can be attributed to factors such as low moisture, acidic pH and high sugar content. Thus, at these concentrations (50%, 75%, 100%) the water content was lesser and did not over dilute the sugar content of the honey giving it an optimum environment for preservation.
In line with the second objective of the research to compare the effect of formaldehyde and honey as a fixative it was observed similarly as by a research by Sabarinath. The rate of fixative diffusion into the tissue and the rate of chemical interactions with different components both affect how quickly an object is fixed. Although the tissues are typically fixed for 24 hours to 48 hours, it is assumed that these processes take at least an hour per millimeter of tissue thickness in practice. Honey fixation in the current study took 24 hours, but the depth of fixative diffusion into the tissue could not be determined due to lack of technical knowledge.
The current study observed that the fixing properties of honey can be attributed to several factors such as acidic pH, low moisture and high sugar content. In honey for example, the presence of antioxidants conveys tissue protection against cellular damage, primarily as a result of the slow release in diluted honey of low concentrations of hydrogen peroxide, a by product of the action of the enzyme glucose oxidase that is present in honey. This deduction was observed in a study by Al- maaini, et al.[13] where he observed that the ascorbic acid, vitamins, carbohydrates, minerals and trace elements contributes to honey’s acidic pH. which is good for nuclear staining.
The study observed that, the presence of the aldehydes in honey is caused by the breakdown of fructose at low pH. The amount of aldehyde present is variable, but because the rate of reaction of the aldehydes is accelerated by heat and storage, it can be measured and used as an indicator of quality. Because honey contains aldehydes, it forms cross links with tissue amino acids in the same way that formaldehyde does. This supports a study by Udonkang and Inyang, where he found that fixatives like honey forms cross links with proteins on tissues.
In a study conducted by Al-maaini, et al.[13] the fixed tissue sections showed satisfactory results in terms of cytoplasmic and nuclear morphology. This study was similar to that observed in the current study where the cytoplasmic and nuclear morphology showed round, smooth and clear nucleus which indicate a satisfactory result. Various studies have been conducted in the past on the effectiveness of honey as a fixative [3,8,10]. The results revealed that honey preserved tissues and showed adequate fixation and preservation of architecture up to 12 hours and beyond of which tissue quality was compromised due to cell swelling and nuclear shrinkage which was observed in 25% concentration of honey in the present study. This was as a result of excess water entering the cells of the tissues causing it to be turgid and easy to disintegrate resulting in poor fixation and necrosis observed from the stained tissue.
Finally it was seen in this study that the quality of fixation with honey did not differ considerably with that of formalin. Though formalin has been proven to be an effective fixative, an adjunct is always beneficial with the current advancements of histotechnology. Honey is readily available in the local market and is neither harmful nor cause health problems with evidence from its long usage.
Conclusion
This study has shown that higher concentrations of 50%, 75% and 100% honey are suitable for long term gross preservation of tissues and further gave excellent nuclear staining of tissues while lower concentrations of 25% gave excellent tissue staining intensity and good preservation for only short term. In comparison to 10% neutral buffered formalin, the staining intensity of honey is deeper due to its dark coloration. Despite aiding in tissue morphology and preservation, the low pH is beneficial because antibacterial agents function well at lower pH.
Limitation
• At 25% concentration after 24 hours, the tissues were turgid and very soft, easily to disintegrate which led to a poor fixation of tissues.
• The micrographs showed high staining intensity of the tissues fixed in honey except 25% concentration due to the honeys deep color imposed on the tissue.
• The depth of fixative diffusion into the tissue could not be determined due to lack of technical knowledge.
Recommandation
• More research should be conducted to resolve honeys cytoplasmic staining issues and the dark coloration associated with the staining intensity of honey.
• More research should be conducted to expose the technicality involved in determining the depth of fixative diffusion of honey.
Conflit of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding statement
There was no specific funding for this work. Laboratory support was provided by the HopeXchange medical Centre.
Acknowledgement
This work was supported by Mr Chris Yaw Asare and Dr Larbi for providing me with laboratory and materials for tissue harvesting.
References
- Onyije FM, Avwioro OG (2012) Excruciating effect of formaldehyde exposure to students in gross anatomy dissection laboratory. Occup Environ Med 3:92-5.
[Google Scholar] [PubMed]
- Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. 6th ed , Elsevier health sciences, United Kingdom, Pp:56-59.
- Sabarinath B, Sundaraman P, Sivapathasundharam B (2020) Honey as a cytological fixative: A comparative study with ethanol. EJMCM 7:22-25.
[Crossref] [Google Scholar] [PubMed]
- Titford ME, Horenstein MG (2005) Histomorphologic assessment of formalin substitute fixatives for diagnostic surgical pathology. Arch Path Lab 129:502-506.
[Crossref] [Google Scholar] [PubMed]
- Mariani-Costantini R, Awadelkarim KD, Barberis M, Clemente C, de Blasio P, et al. (2011) Building sustainable capacity for disease diagnosis in sub-saharan Africa: Case studies of cooperation in diagnostic pathology. InNew Knowledge in a New Era of Globalization 2011 Aug 1. IntechOpen.
- Kwakman PH, Zaat SA (2012) Antibacterial components of honey. IUBMB life 64:48-55.
[Crossref] [Google Scholar] [PubMed]
- Al-Maaini R, Bryant P (2006) The effectiveness of honey as a substitute for formalin in the histological fixation of tissue. J Histotechnol 29:173-176.
[Crossref] [Google Scholar] [PubMed]
- Udonkang MI, Ubi KA, Inyang IJ (2018) Honey as fixative and safer substitute for formalin in histology. Int J Med Lab Res 3:11-17.
- Lalwani V, Surekha R, Vanishree M, Koneru A, Hunasgi S, et al. (2015) Honey as an alternative fixative for oral tissue: An evaluation of processed and unprocessed honey. J Oral Maxillofac Pathol 19:342.
[Crossref] [Google Scholar] [PubMed]
- Ozkan N, Salva E, Cakalagaoglu F, Tuzuner B (2012) Honey as a substitute for formalin? Biotech. Histochem 87:148-153.
[Crossref] [Google Scholar] [PubMed]
- Shobhita KC, Shyam ND, Kumar GK, Narayen V, Priyanka M, et al. (2020) Stereomicroscope as an aid in grossing and histopathological diagnosis: A prospective study. J Oral Maxillofac Pathol 24:459.
[Crossref] [Google Scholar] [PubMed]
- Theresa R, Harsha M, Amberkar VS (2018) Grossing of tissue specimens in oral pathology- Elemental guidelines. Int J Oral Health Sci 8:63.
- Al-Maaini R, Bryant P (2008) Honey as an alternative to formalin in the demonstration of connective tissue components. J Histotechnol 31:67-72.
Citation: Bright OA, Albert OS, Michael AK, Perez Q, Dorcas OO, et al. (2023) A Comparative Study of the Fixative Properties of Honey and Formalin. Diagnos Pathol Open 8:212. DOI: 10.4172/2476-2024.1000212
Copyright: © 2023 Bright OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3219
- [From(publication date): 0-2023 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 2833
- PDF downloads: 386