Research Article Open Access
A Comparative Pharmacokinetic study of Aspirin Suppositories and Aspirin Nanoparticles Loaded Suppositories
| Ravi Sankar V1*, Dachinamoorthi D2 and Chandra Shekar KB3 | |
| 1Department of Pharmaceutics, C.E.S College of Pharmacy, Kurnool, India | |
| 2Department of Pharmaceutics, Q.I.S College of Pharmacy, Ongole, India | |
| 3Department of Chemistry, J.N.T.A University, Anantapur, India | |
| Corresponding Author : | V. Ravi sankar Department of Pharmaceutics C.E.S College of Pharmacy Kurnool-51812, Andhra Pradesh, India Tel: +919494422695 E-mail: ravisankarpharma@gmail.com |
| Received November 24, 2012; Accepted November 28, 2012; Published December 04, 2012 | |
| Citation: Ravi Sankar V, Dachinamoorthi D, Chandra Shekar KB (2012) A Comparative Pharmacokinetic study of Aspirin Suppositories and Aspirin Nanoparticles Loaded Suppositories. Clinic Pharmacol Biopharm. 1:105. doi:10.4172/2167-065X.1000105 | |
| Copyright: © 2012 Ravi Sankar V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
The main objective of the present work was to study the pharmacokinetic parameters and safety of the optimized aspirin suppositories and aspirin nanoparticles loaded suppositories and comparison both. ASA-nanoparticle prepared based on ionicgelation mechanism and optimized Fa9 formulation was isolated. ASA-suppositories prepared for the selection of best base composition and FS2, FS4, FS9, FS11 were selected for incorporation. Selected Fa9 formulation was incorporated into the selected based compositions based on fusion method and evaluated for various evaluation tests. The optimized formulation shows the drug release non-fickian type. Pharmacokinetic models were designed in rabbit model. Method developed in high performance liquid chromatography in rabbit plasma linearity was observed with r2=0.999 in sodium chlorite buffer pH 2.5, acetonitrile, and isopropyl alcohol flow rate of 2ml/min, retention time of 5.310 minutes was observed. Same validate method was used for the determination of various pharmacokinetic parameters Area under curve (AUC0-t), Area under zero infinity (AUC0-infinite), Cmax, Tmax, half life, elimination rate constant, Mean residence time. The histological examination was performed for four weeks at the end of each week animal was scarified and rectal tissue was isolated for histological examination. The comparative kinetics indicates the increase in Tmax, T1/2, AUC0-t and AUMC0-infinite, MRT in the ASA-nanoparticles loaded suppositories when compared with ASAsuppositories. Morphological examination revealed the histological changes such as ulceration and necrosis due to continuous use of ASA-suppositories. ASA-nanoparticles loaded suppositories proved to safe and effective with no observed histological changes.
| Keywords |
| Aspirin suppositories; Aspirin nanoparticles; Aspirin nanoparticles loaded suppositories; Aspirin suppositories pharmacokinetics; Aspirin suppositories histological examination |
| Introduction |
| Aspirin chemically is acetyl salicylic acid (ASA) which is analgesic and antipyretic activity. ASA suppositories are in wide usage in regulatory market. A part from the above pharmacological uses ASA has known for its antiplatelet aggregations due to the inhibition of release of prostaglandins, and has as anti rheumatic and antiinflammatory activity. Aspirin produces analgesic effect of both peripheral and Central Nervous System (CNS) effect. Other then these ASA now days has been explored for new applications like anti platelet aggregation and the present day researches revealing the usage of aspirin with a minimal dosage of 100 mg per day will reduce the carcinoma these finding creates the ASA as interesting subject of study. The oral route suffers with disadvantages such as nausea, vomiting. Rectal route provides the alternative choice for the oral route. Suppositories are solid dosage forms prepared by using fatty base or water soluble base. Water soluble bases more hydrophilic increase the bioavailability. Different factors influencing formulation bioavailability of Indomethacin suppositories in dogs and rabbits were performed by showed a different bioavailability when administered two different marketed formulations in dogs and similarity was observed in rabbits [1]. Mucoadhesive ketorolac tromethamine suppositories formulate was evaluated for its hepatotoxicity with no gastrointestinal tissue damage when compared the usage through oral route [2]. The effect of shape rectal administered suppositories was evaluated for systemic bioavailability studies conducted indicates the importance of shape [3]. Many a research conducted proved that suppositories are better bioavailable compared to oral formulations [1,4,5]. |
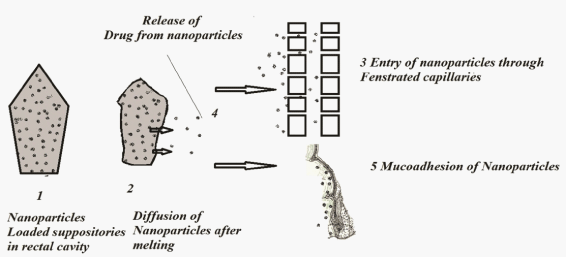
| The ASA-suppositories suffers with irritation and continuous usage leads to ulceration at the rectal site. Present investigative work aimed to reduce the irritation by reducing the direct contact of the ASA to the rectal mucosa. The mechanism involved in nanoparticles form the suppositories was explained in five steps. Nanoparticles loaded suppositories when inserted in to the rectal cavity the suppository will melt at the physiological temperature which will release the nanoparticles in to the cavity represented in Figure 1. The mechanism involved was explained in four steps. 1. Delivery of suppositories to rectal cavity 2. Dissolution (release of nanoparticles) by the rectal fluids 3. The nanoparticles will enter in fenestrated capillaries 4. Diffusion of drug from nanoparticles. 5. Nanoparticles whose size is restricted to the entry of fenestrated capillaries due to the mucoadhesive character nanoparticles will adhere to mucosa of rectum for a prolonged period of time and release the drug in controlled manner. |
| The aim of the present work is to formulate ASA suppositories were prepared by with various compositions of gelatin and glycerin was varied and the best base composition was separated based on in-vitro dissolution study. ASA-nanoparticles by using ionicgelation mechanism and to evaluate the effect of Sodium Tri Poly Phosphate (STPP) and concentration of chitosan. Best optimized formulation of nanoparticles selected and added to the previously selected glycerogelatin base and evaluated for comparative bioavailability study of ASA-suppositories and ASA-nanoparticles loaded suppositories in rabbits. |
| Materials and Methods |
| Materials |
| Chitosan (degree of deacetylation of 88.40% with viscosity of 175 cps) was provided as gift sample from Indian see foods, Ltd, Cochin, Kerala, India. Sodium tripolyphosphate (anhydrous), sodium Chlorate, Sodium hydroxide and glycerinated gelatin was purchased from LOBA Chemie, (Pvt) Ltd., and India. Aspirin 99% purity was obtained as a gift sample from Hetro pharmaceuticals (Pvt) Ltd., India. Acetonitrile and Methanol used are of HPLC grade, purchased from Ranbaxy Fine chemicals Ltd. Mohali. India. De-ionized water was procured from Millipore, India. All the chemicals and other reagents were received as analytical grade were used, Bath sonicator used for degassing (PCI, Analyticals, Mumbai, India). pH meter used for the preparation of chlorate buffuer (Elico). REMI R-2 research centrifuge was used for the separation of plasma from blood. Magnetic stirrer was used for dissolution assemble (RMEI, Electrotechink, Ltd, Vasai, India). |
| Formulation of ASA-nanoparticles |
| Aspirin loaded nanoparticles were prepared by the previously discussed method the chitosan solutions of various concentrations 0.4% to 2% (mg/mL) were prepared (mg/mL) were prepared as shown in (Table 1) by dissolving chitosan in (1% w/w) acidified water which was previously prepared by dissolving in acetic acid at room temperature at overnight stirring. Aspirin was added to the previously prepared chitosan solutions. STPP was dissolved in deionized water to prepare 1 mg/mL concentration. The chitosan solution now added drop by drop in equal volume in to the prepared STPP solution. A nanoparticle starts forming spontaneously by ionic gelation mechanism [6,7]. |
| Formulation of ASA-suppositories |
| Aspirin suppositories were prepared as discussed in literature [8] using glycerogelatin base by using standard fusion method the concentration of glycerin(60%, 67%, 71%, 74%, 76%, 79%), and gelatin, (8%, 11%, 14%, 17%, 22%, 27%) composition were varied from each formulation were prepared displayed in Table 2. Based on displacement value, calculated quantity of micronized aspirin was added to the melted glycerogelatin base at 60°C over heat controlled water bath. The base was kept under stirring in a stainless steel dish, later the base was cooled to 40°C and poured in to the pre calibrated metallic mould and kept for solidification over night in a refrigerator. |
| Preparation of ASA-nanoparticles loaded suppositories |
| Nanoparticles loaded in to the suppository based procedure explained in literature [9]. Based on the previously performed in-vitro characterization studies for aspirin nanoparticles (Fa9), aspirin suppositories best formulations (Table 2) and selected base composition were selected considering displacement value, accurately weighed quantity of 100 mg of aspirin nanoparticles added to the melted glycerogelatin base at 60°C over the thermostatic water bath stirred with glass rod to have content uniformity. The melted glycerogelatin base was cooled to 40°C poured in to the pre lubricated metallic mould and kept in refrigerator for overnight. |
| In-vitro drug release aspirin nanoparticles |
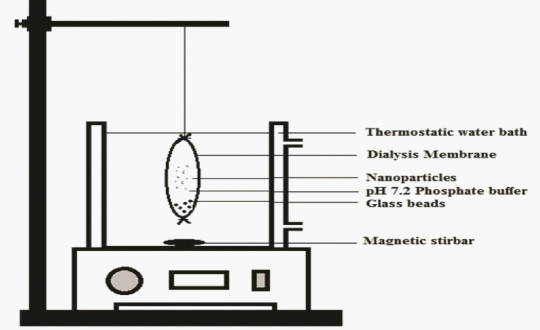
| In-vitro drug release of aspirin nanoparticles were performed as discussed in literature [10] in phosphate buffer at pH 7.2. The nanoparticulate suspension is resuspended in pH 7.2 phosphate buffer and placed in dialysis bag (molecular weight was cutoff 10 kDa). 5 mL of dissolution medium was added in to the dialysis bag and both ends of the bag was sealed and bag was placed in receptor compartment containing 50 mL of pH 7.2 phosphate buffer at 37 ± 5°C over a magnetic stirrer with 50 rpm the assemblage was shown in Figure 2. At periodical time interval, the samples were withdrawn from the receptor compartment the same in quantity of fresh buffer was replaced in order to maintain sink conditions and the samples were analyzed using High Performance Liquid Chromatography (HPLC) form the previously validated method. |
| In-vitro release study of ASA-suppositories and ASA-nanoparticles loaded suppositories |
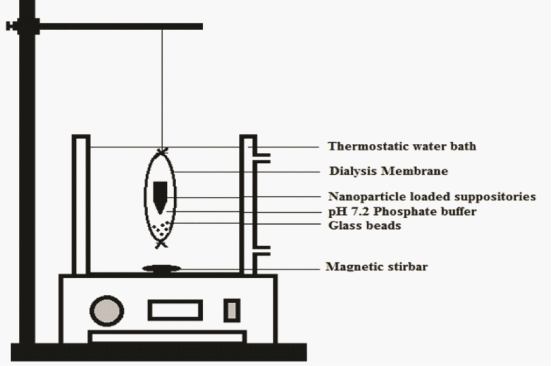
| In-vitro dissolution of ASA-suppositories and ASA-nanoparticles loaded glycerogelatin suppositories was performed as discussed in literature [10]. Suppositories were placed in dialysis bag whose molecular weight was cut 10 kDa and 5 mL of dissolution medium pH 7.2 phosphate buffer was added in to the bag and sealed and the bag was placed in the receptor compartment containing 50 mL of phosphate buffer at 37 ± 5°C over a magnetic stirring at 50 rpm samples were withdrawn at regular intervals and replaced with equal amount of buffer for a period of 1 h ASA-suppositories and 24 h in case of ASA-nanoparticles loaded suppositories. The assemblage was designed as shown in the represented in Figure 3. |
| Chromatographic system |
| Shimadzu HPLC system was containing of CBM-20A, Prominence® series, two pumps of Liquid chromatogram-10 AT VP with SPD-10A VP detector at 227 nm, installed with class-VP software for data acquiring and quantification of peaks. BDS Hypersil® C18 column of length (150 mm×4.6 mm, 5 μm (waters, Mumbai, india) used for chromatographic separation. The samples were filtered through Ultipore® N66 0.2 μm and 0.45 μm membrane filters respectively. |
| Preparation of mobile phase |
| Mobile was filtered sodium chlorite buffer pH 2.5, acetonitrile, and isopropyl alcohol in (85:1:14). The mobile phase mixed was degassed for a period of 10 minutes and filtered through 0.45 μm nylon membrane. The flow rate 2 ml/min and the column were maintained at 25°C. Injection volume was fixed for 20 μl. Total run time was fixed to 10 minutes. |
| Preparation of stock solution |
| 1000 μg/ml was prepared by dissolving 100 mg of aspirin and transferred in to 100 ml volumetric flask and dissolved in 0.1% orthophosphoric acid. The prepared was sonicated for 10 minutes and aliquot of 1 ml, 2 ml, 4 ml, 6 ml, 8 ml and 10 ml stock solution was transferred and diluted with 0.1% orthophosphoric acid and acetonitrile in (50:50) ration and filter through 0.45 μm nylon membrane filter to produce 10 μg/ml, 20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml and 100 μg/ml. |
| Extraction procedure and analytical method validation in rabbit plasma |
| The ASA was added to little quantity of methanol and added to the freshly withdrawn blank rabbit plasma; 100 mg of drug was initially dissolved in 100 ml of methanol. 20 μl of the above prepared stock was added to the 180 μl rabbit plasma to produce different concentrations of 0.5, 2.5, 5.0, 10 and 25 μg/mL and injected into the RP-HPLC system. The 50 μl of blank plasma was kept as standard. Calibration curve was developed based on the values produced on repeated injections for three days both day and night conditions to check the reproducibility an average of 15 injections. The plasma samples were kept at -20°C for three days these concentrations were used to produce the calibration curve and analyzed for slope, linearity, regression, intraday, interday, accuracy, recovery, Limit of quantification (LOQ) and Limit of detection. |
| Study design |
| The present study was approved by the Institutional Animal Ethical Committee (IAEC) licence no: 1035/ac/09/cpcsea. Fifteen New Zealand white male rabbits, weigh 2.5 kg to 3 kg divided in to five groups three animals in each group, were housed individually in standard cases and left for stabilization for one week. Rabbits were fasted for 24 h before administration of suppositories but allowed for free access to water. Suppositories were administered into the rectum 4 cm above the anus through sonde needle. Under this study one groups of receive immediate release ASA-suppositories (Fs9) prepared as explained in the procedure earlier. The last group receives the optimized formulation of Fas9 formulation which is ASA-nanoparticle loaded suppositories with a dose of 10 mg/kg. |
| 2 ml of the sampling was done through the peripheral ear vein prior to drug administration and for first four groups sampling was done at 5, 10, 15, 30, 45, 60 minutes. In case of last group sampling intervals includes 0.5, 1, 2, 4, 6, 8, 12, 14, 16, 20, 24 h. Samples were transferred into test tubes containing heparin. The plasma was separated after centrifugation at 5000 r.p.m for a period of 30 minutes. |
| Area under curve (AUC0-t), Area under zero infinity (AUC0-infinite), Cmax, Tmax, half life and elimination rate constant. All the results were expressed in mean ± SD. |
| Periodical histological examination of rabbit rectum |
| Histological study was carried at periodical intervals for four weeks. The animals are made to receive each one ASA-suppositories and ASA-nanoparticles loaded suppositories per day at the end of the first week first group of animals were scarified fallowed by successive groups in first, second, third and fourth week. The scarified animal liver and rectum were isolated, rinsed, with saline solution and stored in 10% carbonated formalin, The rectal tissues were embedded in paraffin wax and cut in 3 μm thickness. The satins were stained with eosin and examined under binocular light microscope [11]. |
| Result and Discussion |
| ASA-nanoparticles prepared based on electrostatic interaction between chitosan and STPP (Poly valiant an ion) evaluated for various characterization tests [11]. Nanoparticles thus formed are cationic with zeta potential ranges from (24.73 ± 0.01 to 47.99 ± 0.06); entrapment efficiency and particle size appears to be increase with increase in chitosan concentration. In-vitro drug release was performed for a period of 24 h, drug release form all the formulations fallowing zero order release with non fickian type of diffusion. At the end Fa9 formulation appears to release maximum amount of drug shown in Figure 4. Thus selected formulation subjected to freeze-dried and used for feature incorporation. |
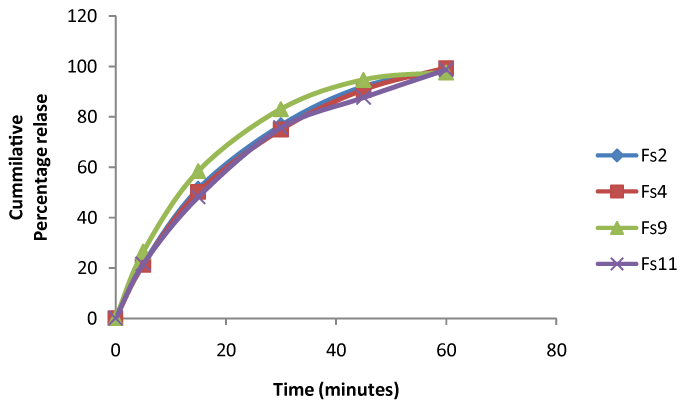
| ASA-suppositories prepared using glycerogelatin as the base, the prepared suppositories was evaluated for the search of best base composition. All the formulation prepared was containing therapeutically equivalent amount of drug (93.5 ± 0.5% to 98.56 ± 0.5%) and lack of fissures, pits. The evaluation tests formed revealed the influence of gelatin and glycerin concentration on the physicochemical properties of aspirin suppositories. It was observed that with increase in gelatin concentration there is decrease in drug release and increase in mechanical strength and other characters. In-vitro drug release study performed for a period of 60 minutes to select the base composition indicates there is no much variation drug the drug release with (98.06 ± 1% to 99.3 ± 0.45%) between the formulation Fs2, Fs4, Fs9, and Fs11 fallowing first order release shown in Figure 5. Selected based composition was used as tool for loading the nanoparticles. |
| Freeze dried nanoparticles of Fa9 formulation was added in to the selected molten glycerogelatin base composition individually and stirred well for uniform distribution and poured in to metallic moulds. The prepared ASA-nanoparticles loaded suppositories evaluated for in-vitro evaluation tests. It was observed that there is sharp decrease in mechanical strength, melting point and disintegration time was seen. This may be due to the disruption of structural integrity in suppositories which was lost due to the addition of nanoparticles during the preparation. All the formulations prepared containing the therapeutic equal percentage of aspirin (74.2 ± 0.8% to 88.9 ± 1.8%). |
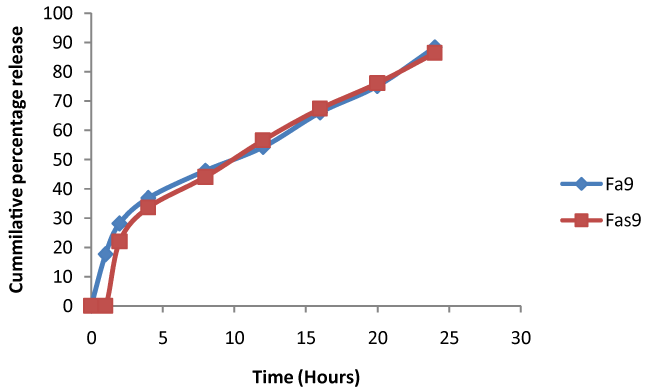
| In vitro release was performed indicates all the formulation appears to release the drug with an initial lag time observed. Fas9 formulation appears to be the best of all formulation with highest percentage of drug release at the end of 24 h (86.4 ± 1.63%). |
| Pharmacokinetic analysis parameters such as Cmax, Tmax, Half life (T1/2), Elimination Rate Const (h-1), AUC0-t, AUC0-inf, AUMC0-t, AUMC0-inf, and MRT (mean residence time). Were determined using PK1 and PK2 excel function for both ASA-suppositories and ASA-nanoparticle loaded suppositories data was displayed in Table 3. |
| From the results it was observed that Tmax of the ASA-suppositories was ranging from 1 ± 0.01 to 1 ± 0.09 indicating there no much variation was seen between the formulations Fs2, Fs4, Fs9 and Fs11. The elimination half life was extended in the aspirin nanoparticles loaded suppositories when compared with ASA-suppositories from 1.43 ± 0.56 to 5.11 ± 0.15. The MRT was extended form 1.41 ± 0.31 to 8.23 ± 0.06 in the optimized Fas9 formulation. Thus aspirin nanoparticle gives a controlled release of drug after released from the suppositories from the mechanism discussed earlier which will improve the bioavailability due to the cationic and mucoadhesive character. |
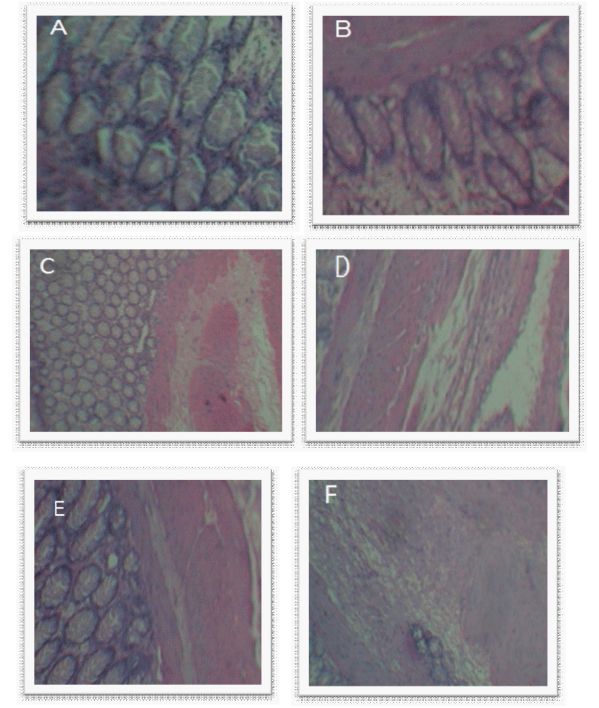
| Periodical histological examination performed for four weeks in rabbit’s, Figure 6(a) represent the control rectal tissue illustrate that epithelium was normal with goblet cells and crypt cells arranged in series can be seen clearly, Figure 6(b) represents the rectal tissue with separated crypt cells and inflamed cells can be seen after one week. The histological examination of successive in second week Figure 6(c), third week Figure 6(d), and fourth in week Figure 6(e) a large number of inflamed cells with ulceration was observed which may be necrosis. |
| This may be due to the continuous localization of ASAsuppositories on rectal wall in addition to the mechanical irritation produced by during insertion of suppositories, Their no much severe changes observed in case of ASA-nanoparticles loaded suppositories as observed on Figure 6(f) with no ulcerative cells, inflamed cells so without any necrosis and internal bleeding was observed. |
| Conclusion |
| The ASA-nanoparticles loaded suppositories can be prepared in glycerogelatin base. It provides the safety and effective use of ASA-suppositories reducing the gastric irritation and increasing the bioavailability for a prolonged use without damaging the rectal tissue. The prepared ASA-nanoparticle loaded suppository provides a delivery tools for drug candidates for effective use. |
| References |
References
- Christian De Muynck, Romain Lefebvre A, Remon JP (1994) The influence of formulation on the relative bioavailability of indomethacin suppositories in dogs and rabbits. Int J Pharm 104: l-10.
- Esmail Mohamed Ramadan, Thanaa Mohamed Borg, Maha Osama Elkayal (2009) Formulation and evaluation of novel mucoadhesive ketorolac tromethamine liquid suppository. Afr J Pharm Pharmacol 3: 124-132.
- Liversidge GG, Nishihata T, Engle KK, Higuchi T (1986) Effect of suppository shape on the systemic availability of rectally administered insulin and sodium salicylate. Int J Pharm 30: 247-250.
- Kirsti Gjellan, Christina Graffner, Stig Strid (1994) Exploratory studies on the in vitro release and bioavailability of dextropropoxyphene from lipophilic and hydrophilic suppositories. International Journal of Pharmaceutics 102: 213-222.
- Noordin MI, Chung LY (2007) Palm Kernel oil blends as Suppository Bases in the delivery of Aspirin. JUMMEC 10: 43-50.
- Calvo P, Remunan-Lopez C, Vila-cpbo JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63: 125-132.
- Oyarzun-Ampuero FA, Brea J, Loza MI, Torres D, Alonso MJ (2009) Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int J Pharm 381: 122-129.
- Pryce-jones RH, Eccleston GM, Abu-Bakar BB (1992) Aminophylline suppository decomposition: an investigation using differential scanning calorimetry. Int J Pharm 86: 231-237.
- Tamer Güner, Mesut Arici, Gökhan Ertan (2004) Preparation and Diffusional Evaluation of Sustained-Release Suppositories Containing Ibuprofen Microspheres. FABAD J Pharm Sci 29: 177-184.
- Caroline Lemarchand, Ruxandra Gref, Patrick Couvreur (2004) Polysaccharide-decorated nanoparticles. Eur J Pharm Biopharm 8: 327-341.
- Sanjay Motwani K, Shruti Chopra, Sushma Talegaonkar, Kanchan Kohli, Farhan Ahmad J, et al. (2008) Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimization and in vitro characterization. Eur J Pharm Biopharm 68: 513-525.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
| 
| 
|
| Figure 1 | Figure 2 | Figure 3 |
| 
| 
|
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 6988
- [From(publication date):
November-2012 - Dec 21, 2024] - Breakdown by view type
- HTML page views : 2563
- PDF downloads : 4425
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
