A Comparative Assessment of the Diagnostic Value of Anti-cyclic Citrullinated Peptide Antibodies and Rheumatoid Factor in Rheumatoid Arthritis
Received: 04-Jan-2014 / Accepted Date: 04-Feb-2014 / Published Date: 06-Feb-2014 DOI: 10.4172/2161-0681.1000158
Abstract
Rheumatoid arthritis is a systemic, chronic inflammatory autoimmune disease affecting many tissues but principally attacking the joints. Auto antibodies such as rheumatoid factor and anti-cyclic Citrullinated peptide antibodies have important diagnostic value. This cross-sectional analytical study was performed at a single medical institution in central Iran (Shahid Sadoughi Hospital, Yazd, Iran) in order to compare the diagnostic value of anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Serum levels of anti-cyclic citrullinated peptide antibodies were determined by enzyme-linked immunosorbent assay, and levels of rheumatoid factor were determined by turbidimetry on a latex-enhanced agglutination assay in 266 patients with rheumatoid arthritis and 134 patients with non-rheumatoid arthritis rheumatic diseases.
Among the 266 patients with rheumatoid arthritis, 188 patients (70.7%) were tested positive for anti-cyclic citrullinated peptide antibodies, and 123 patients (46.2%) were tested positive for rheumatoid factor. The sensitivity, specificity, positive predictive value and negative predictive value of anti-cyclic citrullinated peptide antibodies for diagnosing rheumatoid arthritis were 70.76%, 85.07%, 90%, and 59% respectively. Those for rheumatoid factor were 46.26%, 90.29%, 90%, and 45% respectively. The anti-cyclic Citrullinated peptide antibodies measurement is a useful test for diagnosing rheumatoid arthritis. Due to its high positive predictive value and negative predictive value, it is an important diagnostic test and gives accurate diagnosis of the disease. However, this does not mean that anti-cyclic citrullinated peptide antibodies can replace rheumatoid factor in the diagnosis of rheumatoid arthritis, because not all rheumatoid arthritis patients have anti-cyclic Citrullinated peptide antibodies. The two tests therefore appear to be complementary.
Keywords: Rheumatoid Arthritis; Citrullinated peptide; Rheumatoid factor; Comparison
313191Introduction
Rheumatoid Arthritis (RA) is a relatively common condition, with a prevalence of approximately 1% [1] Conventionally, the serology test routinely used in RA is the determination of serum Rheumatoid Factor (RF) which has high and acceptable sensitivity, but modest specificity, particularly in the early course of the disease [2]. The more recent auto antibodies for the diagnosis of RA are anti-cyclic citrullinated peptide antibodies (anti-CCP antibodies). Anti-CCP testing is particularly useful in the diagnosis of RA and it is able to predict the severity of the disease and the irreversible damage [3]. Anti-CCPs have recently been added as one of the criteria in the 2010 American College of Rheumatology (ACR) /European League Against Rheumatism (EULAR) classification of RA [4]. Some studies have shown that anti- CCP antibodies are moderately sensitive but highly specific for the diagnosis of RA, and their specificity is higher than RF [5]. It is claimed that the presence of anti-CCP antibody in a patient could be the sign of RA with a rate of 90-95% [6].
Through a meta analysis of 78 studies, Nishimura et al. [7]. Revealed the pooled sensitivity and specificity of 67 and 95% for anti-CCP and 69 and 85% for RF and they reported that anti-CCP was more specific than RF for diagnosing RA [7]. It seems that ethnic differences in addition to differences in study design may contribute to the discrepancies in the literature on the utility of RF and anti-CCP for diagnosis of RA. Even in a country such as Iran, there is variation in results related to sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of these two tests. For example, Moghimi et al. [8] showed that the overall performance of the anti-CCP and RF tests for differentiating RA and other inflammatory polyarthritis were similar [8]. Meanwhile in another research Aflaky et al. [9] revealed that AUC (Area under the Curve) for anti-CCP was higher than that for RF and concluded that anti-CCP might be of a better diagnostic value than RF test [9]. On the other hand, Abolghasemi et al. [10] offers a combination of these tests rather than anti-CCP or RF to get the best results in RA diagnosis and prognosis [10].
Therefore, we conducted a cross-sectional study to identify the diagnostic value of anti-CCP antibodies and RF in patients with RA at a referral community hospital in central Iran. We have also studied the presence of anti-CCP antibodies in RF negative patients with RA.
Materials and Methods
The study was approved by the university ethics committee, all the participants were informed about the research, and informed consents were obtained. In this cross-sectional study we studied 400 serum samples: 266 from definite RA patients according to the American College of Rheumatology (ACR) criteria [4], (228 women and 38 men; mean age 45.5 ± 13.8 years; range, 8-74 years) and consecutively recruited from the rheumatology out-patient clinic of Shahid Sadoughi Hospital of Yazd, Iran. 198 (61%) of these patients were classified as having early RA because their symptoms’ onset, had appeared <1 year before this study and radiological examinations revealed no lytic lesions in the wrists, hands, and feet. To provide data on assay specificity, 134 controls (matched for age and sex including 22 males and 112 females with non-RA rheumatic diseases) selected on the basis of their clinical diagnosis, were also studied.
Anti-CCP antibodies were tested by first-generation ELISA (AESKULISA). The anti-CCP was considered positive at values greater than18 U/ml. The RF was measured by turbidimetry on a latexenhanced agglutination assay (Roche Integra, Penzberg, Germany). The RF was considered positive at values greater than 10 U/ml. Each of these tests was performed and evaluated by operators who were blinded to other serological results and unaware of the patients’ clinical data. The sensitivity and specificity for each assay was determined. Our study was not designed to investigate the relationship between clinical symptoms and laboratory results. The statistical analysis was performed using SPSS, version 16 (SPSS Inc., Chicago, IL, USA). Frequency table was used to assess sensitivity, specificity, negative and positive predictive values. 95% confidence interval was calculated using the Wilson method.
Results
The demographic data are summarized in Tables 1 and 2.
| RA | Positive | Negative | Sum | |||
|---|---|---|---|---|---|---|
| Sex | Number | percent | Number | percent | Number | percent |
| Female | 228 | 67.1 | 112 | 32.9 | 340 | 100 |
| Male | 38 | 63.3 | 22 | 36.7 | 60 | 100 |
| Sum | 266 | 66.5 | 134 | 33.5 | 400 | 100 |
Table 1: Demographic information according to gender.
| RA | Positive | Negative | Sum | |||
|---|---|---|---|---|---|---|
| Age groups | Number | percent | Number | percent | Number | percent |
| Aug-34 | 74 | 68.5 | 34 | 31.5 | 107 | 100 |
| 35-44 | 67 | 69 | 30 | 31 | 97 | 100 |
| 45-54 | 77 | 62.6 | 46 | 37.4 | 123 | 100 |
| 55-74 | 48 | 65.7 | 25 | 34.3 | 73 | 100 |
| sum | 266 | 66.5 | 135 | 33.5 | 400 | 100 |
Table 2: Demographic information according to age.
Anti-CCP
Based on the cutoff value suggested by the manufacturer, among 266 patients with RA, 188 sera were positive for anti-CCP at >18 unit/ml (Table 3). The sensitivity was 70.67% (CI: 64-75%). In 134 participants without clinical diagnosis of RA only 20 sera (9.61%) were positive. The specificity was 85.07%. (CI: 78-90%).
| RA | Predictive value | |||
|---|---|---|---|---|
| Positive N (%) | Negative N (%) | |||
| Anti-CP | Positive | 188(70.67%) | 20(14.93%) | 90.38%1 |
| Negative | 78(29.33%) | 114(85.07%) | 59.37%2 | |
1Positive predictive value.
2Negative predictive value.
Sensitivity=70.67%
Specificity=85.07%
Table 3: Relative distribution of anti-CCP results in studied patients according to RA diagnosis.
RF
Based on the cutoff value suggested by the manufacturer in the RA group comprising of 266 patients, 123 sera were positive for RF at >10 IU/ml. The sensitivity was 46.24% (CI: 40-52%). In the non-RA group (134 participants), 13 sera were positive for RF at >10 IU/ml. The specificity was 90.29% (CI: 84-94%) (Table 4). Among RF-positive RA patients, 95 of 123 patients (77.2%) were anti-CCP antibodies positive. Among RF-negative RA patients, 93 of 143 patients (65%) were anti- CCP antibodies positive. In the non-RA groups, 3 samples (23.1%) were both RF and anti-CCP positive, while 10 (76.9%) were only RF positive and 17 (14%) were only anti-CCP positive.
| RA | Predictive value | |||
|---|---|---|---|---|
| Positive | Negative | |||
| N (%) | N (%) | |||
| RF | Positive | 123(46.24%) | 13(9.71%) | 90.44%1 |
| Negative | 143(53.76%) | 121(90.29%) | 45.83%2 | |
1Positive predictive value.
2Negative predictive value.
Sensitivity=46.24%
Specificity=90.29%
Table 4: Relative distribution of RF results in studied patients according to RA diagnosis.
The anti-CCP antibodies had PPV and NPV for diagnosis of RA of 90.38% (CI: 85-93%), and 59.37% (CI: 52-66%) respectively. Those for RF were 90.44% (CI: 84-94%), and 45.83% (CI: 39-52%) respectively.
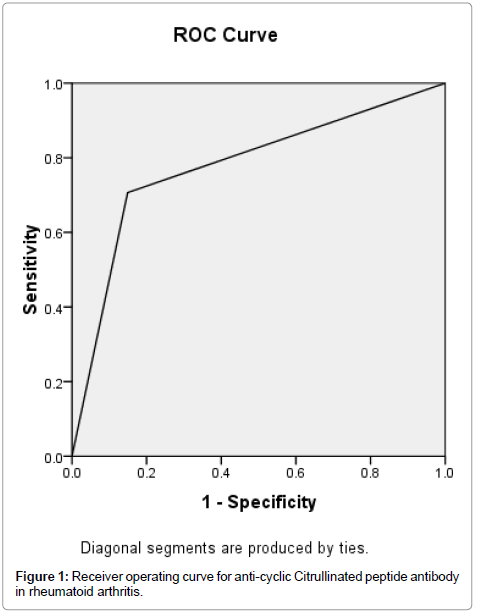
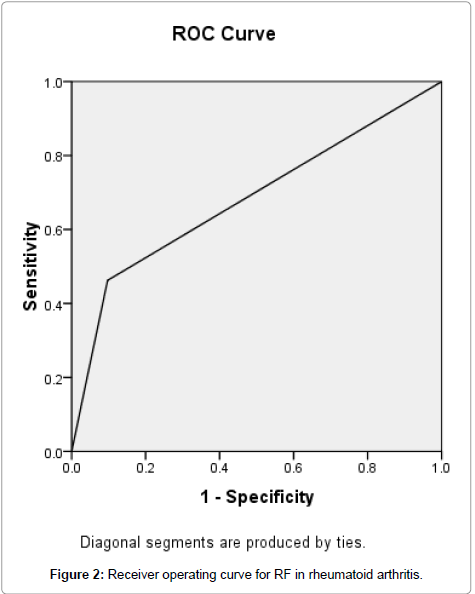
Receiver Operating Characteristic (ROC) curve was generated by the method of Metz for anti-CCP, and its accuracy was measured by the area under the ROC curve. The area under the curve for anti-CCP was 0.779. The accuracy was therefore considered as good (Figure 1), but the area under the curve for RF was 0.683 and the accuracy was considered as fair (Figure 2).
Regarding the anti-CCP titer, 96% of cases with titer higher than 201 (201-1500) had definite RA whereas, 55.4% of patients with titer less than 101 (0.2-101) had definite RA. Among patients with early RA disease, 138 (69.7%) cases were positive for anti-CCP and 84 (42.4%) cases were positive for RF. Among patients with symptoms lasting more than one year, 47 cases (78.3%) had positive anti-CCP results and 35 (58.3%) patients were positive for RF test.
Discussion
The present study compared diagnostic performances of these two tests in RA patients in comparison to those in non-RA rheumatic diseases. Recent papers have highlighted the importance of early treatment of RA [11]. Markers such as RF and anti-CCP antibodies were selected from among other markers. RF has been widely used as a screening test for patients with arthritis. RF is Prognostically useful and one recent study revealed that RF titer reflected RA disease activity [12] RF constitutes one of the classification criteria proposed by the American College of Rheumatology (ACR). But RF is present in patients with other autoimmune and infectious diseases, and even in a noticeable proportion of normal healthy subjects, particularly in old individuals [13]. More recently anti-CCP has been described for RA. About 35–40% of the RF-negative patients are anti-CCP antibodypositive. Anti-CCP antibodies have also demonstrated prognostic utility with regard to radiographic outcomes [14]. In our study the sensitivity and specificity of RF were 46.2 and 90.29%, respectively. These values are similar to those reported by Aflaki using latex fixation test [9] but are different from Hodkinson’s study [15]. A literature review shows that the sensitivity of RF in RA has ranged from 26 to 90 percent [16]. A possible explanation for this wide range of sensitivity could arise from racial and geographical differences. In two studies from Turkey, the sensitivity of RF in Turkish patients with RA was 43% and 40.7% [17,18]. In a recent study conducted by Abdul Wahab et al. [19] in Malaysia, the sensitivity of RF was calculated at 43%. Again these values are similar to or lower than our results. The sensitivity or specificity of RF does not appear to be affected by the use of latex agglutination that was used in this study. One study has reported similar specificity and sensitivity for RF assessed either through latex fixation or ELISA [20]. Therefore, it is unlikely that the use of latex agglutination test in the present study significantly contributed to the observed lower sensitivity. There do appear to be genetic influences on RF seropositivity [21]. In addition environmental factors (such as cigarette smoking) are possibly important in RF production [22]. In the current study 57.6% of patients with early RA had negative RF results. Although negative RF results are consistent with conditions other than RA, they do not rule out RA. Because RF-negative patients may seroconvert, a follow up testing during the first year of disease may be useful. In this study the anti-CCP antibody test was positive in 110 cases (41.7%) of seronegative RA patients. In this study, we found that RF was positive in only 58.3% of the patients with established disease. This might be due to treatment. Some studies documented that the level of the RF decreases with treatment [23,24]. The current study showed that RF was also positive in 13 patients with non-RA rheumatic diseases. These findings are similar to those of a previous study which revealed RF was positive in many other rheumatic and non-rheumatic diseases [13]. Although it is claimed that as a diagnostic test, RF has modest specificity, particularly in the early course of the disease [2], in the present study its specificity was 90.29%. Our study showed that anti- CCP had a sensitivity of 70.67% and specificity of 85.07%. The sensitivity was similar to that reported by Maraina [25] but more than that reported by Sharif et al. [26]. The specificity of anti-CCP in the present study was somehow lower than that reported by Maraina [25] but similar to that of Sharif et al. [26]. To explain the differences between reported sensitivity and specificity of anti-CCP in different studies, it must be considered that1- anti-CCP antibodies are a heterogeneous group of antibodies directed against different epitopes on the citrulline molecule. Each patient’s serum contains different subsets of antibodies. The synthetic peptide used in this essay represents a relatively small set of antigenic determinants that do not entirely encompass the antigenic determinants present on the as yet unknown antigenic molecule in the joint [27]. 2-In addition in the laboratory assessment, we used the first generation of anti-CCP-1 kit, because anti-CCP-2 kit was not available at the time of this study in our city. However, the first-generation anti- CCP assay has a low analytical sensitivity (ranging from 48 to 68%) [28]. 3-One study showed that the specificity and sensitivity of anti- CCP antibodies may depend on the patient’s race [29]. 4-Another explanation for the discrepancy is that the differences in the patient populations (especially disease duration) among these studies might have influenced the results. For example, in the study of Kamali et al. [18] the sensitivity of the anti-CCP antibody for RA is higher than ours. Their patients had at least 1 year of disease duration, in contrast to the symptom duration of less than one year in more than 60% of our patients. Nevertheless, in this study it was interesting to evaluate anti-CCP behavior in RA patients in relation to the duration of disease. In patients with early arthritis the correlation with anti-CCP was significant, thus indicating that this assay may be used even in the early phases of the disease. 5-Specificity in each diagnostic test is negatively related to the frequency of false positive results. So differences related to specificity of anti-CCP might be due to frequency of positive anti-CCP results in non-RA patients. The high specificity of anti-CCP assays in some studies did not exclude some false positive results. Some patients with various non-RA diseases demonstrated high anti-CCP titers. In one recent study, Li et al. investigated RF and anti-CCP positivity in a total of 1,018 healthy donors, 212 patients with RA, and 435 patients with other connective tissue disease, and they found out that anti-CCP was present in 2.6% and RF was present in 21.5% of the healthy donors [30]. In our study the PPV of anti-CCP was 90.38% and PPV of RF was 90.44%. The NPV of anti-CCP was 59% and NPV of RF was 45%. It should be noted that the value of any diagnostic test has been related to its disease prediction ability. Positive predictive value in diagnostic studies is related to disease prevalence and thus in population with lower prevalence of that disease, we expect to have lower PPV. In one study it was noted that anti-CCP positivity was significantly higher in RA patients with severe joint destruction than those with minimal joint destruction [31]. Although in this study we could not evaluate this parameter, we found out that the diagnosis of RA was more accurate for higher anti-CCP titers.
Conclusion
This study showed that anti-CCP antibodies indeed are good serological markers for RA. Anti-CCP antibodies are well suited as a front line diagnostic test for RA and especially early RA. In RF seronegative patients, anti-CCP can be helpful in confirming the diagnosis of RA. Step up of anti-CCP testing in the ACR’s updated 2010 RA classification criteria reveals the clinical value of these tests for the diagnosis of RA patients. However, this does not mean that anti- CCP can replace RF in diagnostic and prognostic testing for RA. We offer a combination of anti-CCP and RF tests rather than anti-CCP or RF to get the best results in RA diagnosis. Considering the ethnic difference, continuous assessment of the diagnostic value of auto antibodies in RA in different ethnic groups is useful and helps revealing the heterogeneous nature of the disease.
References
- Vinay Kumar, Abul K Abbas, Jon C Aster (2013) Bones, Joints, and Soft Tissue Tumors. In: Robbins Basic Pathology. (9thedn), Saunders, Elsevier, USA, 784.
- Miriovsky BJ, Michaud K, Thiele GM, O'Dell JR, Cannon GW, et al. (2010) Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 69: 1292-1297.
- Hayashi N, Kumagai S (2010) [Anti-cyclic citrullinated peptide antibodies and rheumatoid arthritis]. Rinsho Byori 58: 466-479.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, et al. (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and rheumatism 62: 2569-2581.
- Lee DM, Schur PH (2003) Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 62: 870-874.
- Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, et al. (2007) Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Annals of internal medicine 146: 797-808.
- Moghimi J, Ghorbani R, Hasani F, Sheikhvatan M (2013) Discriminative and diagnostic value of anti-cyclic citrullinated peptide antibodies in Iranian patients with rheumatoid arthritis. Rheumatol Int 33: 601-605.
- Aflaky E, Shenavandeh S, Ashraf MJ (2010) A comparison of performance of anti-cyclic citrullinated peptide 2 and citrullinated protein antibodies in the diagnosis of rheumatoid arthritis in Iranian patients. Rheumatol Int 30: 461-466.
- Abolghasemi S, Gitipour A, Morteza A (2013) The sensitivity, specificity and accuracy of anti-citrulline antibody test in diagnosis of rheumatoid arthritis. Rheumatol Int 33: 1027-1030.
- Klareskog L, Catrina AI, Paget S (2009) Rheumatoid arthritis. Lancet 373: 659-672.
- Geng Y, Zhou W, Zhang ZL (2012) A comparative study on the diversity of clinical features between the sero-negative and sero-positive rheumatoid arthritis patients. Rheumatol Int 32: 3897-3901.
- Westwood OM, Nelson PN, Hay FC (2006) Rheumatoid factors: what's new? Rheumatology (Oxford) 45: 379-385.
- Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, et al. (2003) Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 62: 120-126.
- Hodkinson B, Meyer PW, Musenge E, Ally MM, Wadee AA, et al. (2010) The diagnostic utility of the anti-CCP antibody test is no better than rheumatoid factor in South Africans with early rheumatoid arthritis. Clin Rheumatol 29: 615-618.
- Shmerling RH, Delbanco TL (1991) The rheumatoid factor: an analysis of clinical utility. Am J Med 91: 528-534.
- Â Ates A, Karaaslan Y, Aksaray S (2007) Predictive value of antibodies to cyclic citrullinated peptide in patients with early arthritis. Clin Rheumatol 26: 499-504.
- Kamali S, Polat NG, Kasapoglu E, Gul A, Ocal L, et al. (2005) Anti-CCP and antikeratin antibodies in rheumatoid arthritis, primary Sjögren's syndrome, and Wegener's granulomatosis. Clin Rheumatol 24: 673-676.
- Abdul Wahab A1, Mohammad M2, Rahman MM3, Mohamed Said MS4 (2013) Anti-cyclic citrullinated peptide antibody is a good indicator for the diagnosis of rheumatoid arthritis. Pak J Med Sci 29: 773-777.
- Karim Raza, Mike Breese, Fimls, Peter Nightingale, Kanta Kumar, et al. (2011) Predictive Value of Antibodies to Cyclic Citrullinated Peptide in Patients with Very Early Inflammatory Arthritis. J Rheumatol.
- Silman A, Ollier B, McDermott M (1988) HLA: linkage with rheumatoid arthritis or seropositivity. J Rheumatol 15: 1189-1192.
- Ian Enzer,Graham Dunn, Lennart Jacobsson, Peter H. Bennett,William C. Knowler, Alan Silman (2002) An epidemiologic study of trends in prevalence of rheumatoid factor seropositivity in Pima Indians: Evidence of a decline due to both secular and birth-cohort influences, Arthritis & Rheumatism 46: 1729-1734.
- De Rycke L, Verhelst X, Kruithof E, Van den Bosch F, Hoffman IE, et al. (2005) Rheumatoid factor, but not anti-cyclic citrullinated peptide antibodies, is modulated by infliximab treatment in rheumatoid arthritis. Ann Rheum Dis 64: 299-302.
- Chen HA, Lin KC, Chen CH, Liao HT, Wang HP, et al. (2006) The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 65: 35-39.
- Maraina CH, Nurdayana AK, Rusni D, Azwany Y (2010) Diagnostic value of anti-modified citrullinated vimentin in rheumatoid arthritis. Int J Rheum Dis 13: 335-339.
- Sharif SK, Eghbal S, Gharibdoost F, Kbarian MA, Shahram F, et al. (2007) Comparative study of anti-CCP and RF for the diagnosis of rheumatoid arthritis. APLAR Journal of Rheumatology 10: 121-124.
- van Jaarsveld CH, ter Borg EJ, Jacobs JW, Schellekens GA, Gmelig-Meyling FH, et al. (1999) The prognostic value of the antiperinuclear factor, anti-citrullinated peptide antibodies and rheumatoid factor in early rheumatoid arthritis. Clin Exp Rheumatol 17: 689-697.
- Bizzaro N, Mazzanti G, Tonutti E, Villalta D, Tozzoli R (2001) Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. Clin Chem 47: 1089-1093.
- Mimori T (2005) Clinical significance of anti-CCP antibodies in rheumatoid arthritis. Intern Med 44: 1122-1126.
- Li T, Bao J, Yin J, Xu HJ (2011) [The specificity of anti-cyclic citrullinated peptide antibodies in the diagnosis of rheumatoid arthritis from a large cohort study in the Chinese]. Zhonghua Nei Ke Za Zhi 50: 99-101.
- Vallbracht I, Rieber J, Oppermann M, Förger F, Siebert U, et al. (2004) Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 63: 1079-1084.
Citation: Binesh F, Salehabadi HS, Behniafard N, Ranginkaman K, Behniafard N (2014) A Comparative Assessment of the Diagnostic Value of Anti-cyclic Citrullinated Peptide Antibodies and Rheumatoid Factor in Rheumatoid Arthritis. J Clin Exp Pathol 4:158. DOI: 10.4172/2161-0681.1000158
Copyright: © 2014 Binesh F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16431
- [From(publication date): 3-2014 - Jul 14, 2025]
- Breakdown by view type
- HTML page views: 11767
- PDF downloads: 4664