A Comparative Approach to Measure Elasticity of Whole Blood by Small Amplitude Oscillation
Received: 18-Nov-2016 / Accepted Date: 23-Dec-2016 / Published Date: 31-Dec-2016
Abstract
In the past, mammalian blood was characterized by flow curves to calculate the dynamic shear viscosity of blood. It was found out that whole blood viscosity (WBV) is very divers among the animal kingdom, predominantly at low shear stresses that allow red blood cell (RBC) aggregation. RBC aggregation might be the most significant factor for blood elasticity as well. To verify this hypothesis, we tested whole blood from species with high (horse), medium (man) and low (sheep) RBC aggregability by small amplitude oscillation in CSS-mode. Blood samples were hematocrit (HCT) adjusted (40%, 50%, 60%) and tested at 7°C, 22°C and 37°C. Storage modulus (G´) increased with RBC aggregability and HCT, but decreased with temperature, as expected. Interestingly, the gradient of the G´-increase with HCT was species-specific. The lower dependency of G´ on the equine HCT value could be a benefit during physical performance when high numbers of RBCs are released from the spleen. In sheep, a HCT-threshold had to be overcome before elasticity of the blood sample could be measured, suggesting that the cohesive forces between RBCs, and between RBCs and plasma molecules must be very low. The frequencies for tests under quasi-static condition were in a narrow range around the physiologic heart rate of the species. In horse, time-dependent influences concurred at frequencies lower than 3 rad.s-1 probably due to sedimentation of RBC aggregates. In conclusion, elasticity of blood depends not only on the amount of blood cells, but also on their mechanical and functional properties.
Keywords: Hemorheology; Blood; Species differences; Sheep; Horse; Small amplitude oscillation; Elasticity; Yield point; Storage modulus
42207Introduction
Rheology identifies the sum of cohesive and adhesive forces within a sample during controlled application of shear in a given geometry. In blood, these forces are given by the quantitative and qualitative properties of blood cells, by the constitution of blood plasma, and by the interaction of blood cells with the plasma proteins at physiological pH and ionic strength. Compared to particle systems or dispersions, the viscosity of whole blood (WBV) is low, even for high cellular fractions such as 40-45%, which is the physiological hematocrit (HCT) of man and most animal species. This is the result of the unique red blood cell (RBC) properties.
For instance, RBCs have a high surface-to-volume ratio that allows their deformation in narrow vessels [1]. Exposure to shear forces causes a shape change from the relaxed biconcave towards folded (f.i. parachute) shapes [2-4]. RBCs exhibit shape memory [5] when entering vascular regions with low flow. The RBC membrane is an active ATP-driven structure. Dynamic fluctuations of the cell membrane are increased in relation to metabolic and thermal energy [6]. RBC membranes use a tank thread motion to absorb hydrodynamic stress - a process that facilitates laminar blood flow and suspension stability within a blood vessel [7]. The tank thread frequency starts at a certain shear stress threshold [8,9] and depends on the viscosity of the suspending medium [10,11]. RBC membranes possess a further dynamic feature that enables a rolling motion in shear flow [9]. This feature should reduce energetically costly deformation.
RBCs form aggregates - called as Rouleaux - at low volume flow [12-15]. These aggregates break up when shear rates increase at higher blood flows. There is remarkable diversity in RBC aggregability among the mammalian species [16]. For instance, RBC aggregation is too low to be measured with routine techniques in cow, sheep, goat, mouse, and rat blood. In contrast, in horse and other equidae, the physiological RBC aggregation is as high as it would be in inflammatory disease in man [17,18]. RBC aggregation affects venous vascular resistance [19], and clinical cases associated with high RBC aggregation indicate specific diseases [20]. The impact of RBC aggregability for in vivo blood flow is discussed controversially. Although there is evidence that high RBC aggregation blunts the parabolic blood velocity profile [21], moderate RBC aggregation seems to promote blood flow [22,23], at least in comparison to non-aggregating RBC suspensions. It is generally agreed that in vivo blood viscosity rises when intravascular shear forces are low enough to allow RBC aggregation. By considering blood flow as Poiseuille flow [24], the lowest wall shear rates are calculated for the postcapillary venules. RBC aggregates have been seen with high-speed video microscopy in the venules after passing the post-capillary region [25]. However, it is not clear if RBC aggregation can occur in resistance vessels. The physiological relevance of RBC aggregation lies in the phase separation phenomenon of composite fluids in narrow tubes reaching a critical diameter. Due to the axial migration of RBCs in the tube flow [26], a marginal cell-free layer (CFL) is formed, whereby the magnitude of the width of this layer (CFLW) is linked to RBC aggregability. It is accepted that this CFL reduces the endothelial shear stress and subsequently, an intrinsic response mediated by vasoactive factors starts to adjust the vascular diameter in arterioles [27,28]. This in turn modulates the distribution of RBCs in subsequent vessels [29-31].
Rheological properties of blood are usually measured by viscosity as a function of shear rate. Methods to test properties of singular RBCs were recently summarized [32]. Blood was characterized as a shear thinning viscoelastic fluid, showing different degree of thixotropy in relation to RBC aggregation. Typically, at low shear rates RBCs aggregate to linear clusters, while at high shear rates RBCs are singularly suspended and elongated by the shear forces [33]. In contrast to rotational flow, small amplitude oscillating shear flow (SAOS) can be used to study the elastic behaviour of fluids under quasi-static conditions. An application of this method to human blood has been described recently [34]. In the present study we tested human, equine and ovine blood. The two animal species were selected due to their contrasting RBC aggregability. Since the HCT of the blood samples was adjusted, the differences of blood rheology was investigated in regard to the mechanical and functional RBC properties of the species. It came out that blood elasticity was pronounced if RBCs have the ability to form super-structures in shear flow.
Materials and Methods
Blood samples
Whole blood of healthy man, horse, and sheep were used for this study. 15 human volunteers (8f, 7m, age: 22-50 years), 5 Warmblut horses (3 mares, 2 geldings, age: 13-26 years), and 11 female Milchschaf sheep (age: 1.2-3.6 years) were used. 90 mL blood was withdrawn into EDTA tubes from each individual by venous puncture (man: V. radialis, horse and sheep: V. jugularis) by a vacutainer system. Samples were centrifuged at 2000 rpm for 3 min and blood plasma was separated. New samples were reconstituted out of RBC concentrate and autologous plasma to generated whole blood samples with HCT values of 40, 50 and 60%. The samples were carried in insulated bags to the laboratory and kept in the fridge prior to their measurement. All measurements were finished within 6 h following withdrawal.
The procedure was approved by the Ethics Committee of the Medical University Vienna (1892/2013) and the Austrian Federal Ministry of Science and Research (animal license number: GZ-1744/115-97/98).
Rheological protocol
SAOS measurements were performed using the stress controlled Physica MCR301 rheometer (Anton Paar, Graz, Austria). 3.5 mL of each blood sample was filled into the stainless steel double gap cylinder system and was analyzed at three different temperatures (7, 22, 37°C) starting with the lowest temperature. Isothermal amplitude and frequency sweep tests were performed.
Strain dependency of blood samples was measured at fixed frequency (10 rad s-1) to compare the yield stresses between the species. In order to carry out the subsequent frequency tests in linear regime, some of the human and horse blood samples were tested at frequencies between 1 and 20 rad s-1. After a pre-shear interval (man and sheep: 30 s rotation at 300 s-1 followed by a 20 s interval at 1 s-1; horse: 30 s oscillation at 0.01 Pa and 10 rad s-1), increasing shear stresses of 0.001- 10 Pa were applied by a logarithmic shear stress ramp. Yield points were estimated by the Rheoplus software (version 4.2, Anton Paar, Graz, Austria).
Frequency sweep tests were performed at 5-10 mPa throughout the whole frequency range (20-1 rad s-1). The blood samples were subjected to frequency sweep from high to low frequency only.
Descriptive statistic was performed by IBM® SPSS® Statistics (version 22).
Results
Amplitude sweep tests
Data of amplitude sweep tests are provided in Table 1.
“Allowed” frequencies for the subsequent frequency sweep tests were within the narrow range between 3 and 15 rad s-1. These frequencies correspond to the range of the physiologic heart rate of the species at rest (0.5-2.5 Hz).
The software easily defined the linear viscoelastic (LVE) range at 7°C in man and horse (as well as in sheep at high HCT: 60%), but became imprecise when the temperature increased. Especially at 37°C, the G´- values were sloping – although continuously decreasing. Occasionally, the LVE range at 37°C had to be determined based on G´´-values that always displayed a plateau until a certain shear stress was reached. As expected, experiments became more reproducible when HCT was high and temperature was low.
Basically, yield points and corresponding G´-values were very low in blood although traceable. In blood samples at 40% HCT and 37°C, yield points are not provided due to sloping of G´. Yield points appeared to be slightly higher in horse compared to man. In sheep, yield points could be determined at 60% HCT only, however, the obtained values were the highest among the three species.
Frequency sweep tests
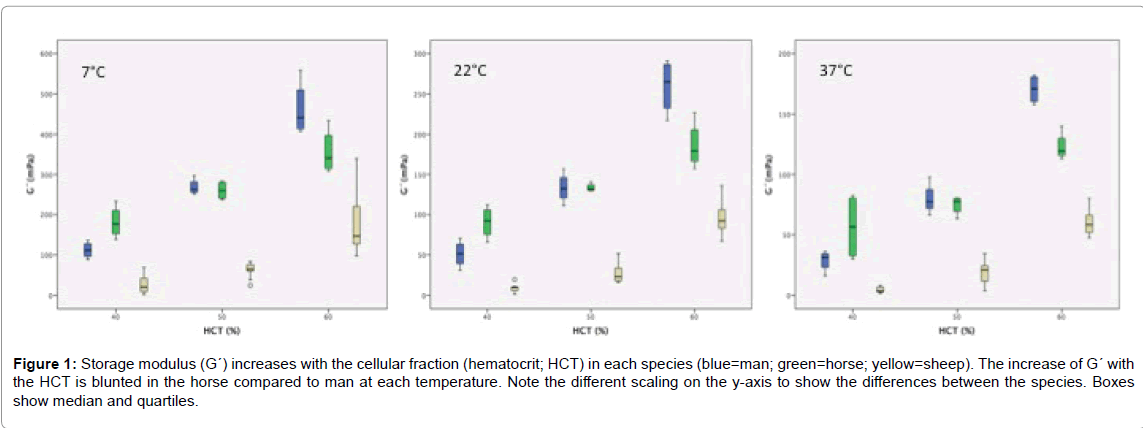
G´-values increased with the HCT and decreased with the temperature (Table 2). At 40% HCT and 37°C the interquartile distance became larger indicating greater variance among the samples. There was no G´-G´´-crossover within the “allowed” frequencies. Sheep with the lowest RBC aggregation showed the lowest G´-values. It is interesting to note that G´ was lower in man than in horse at 40% HCT, but higher in man than in horse at 60% HCT at each temperature. At 50% HCT the median values of man and horse were nearly identical (Figure 1). Loss factor (tan δ) decreased with the HCT and increased with the temperature in each sample. The lowest loss factors: 1.80 (1.58/1.82) were measured in horse at 60% HCT and 7°C, verifying blood of these respective species as viscoelastic fluid even at fridge temperature.
| ty at 7°C (in mPa) | 40% HCT | 50% HCT | 60% HCT |
|---|---|---|---|
| Man | 21 (8/22) | 33 (13/46) | 46 (45/46) |
| Horse | 33 (20/86) | 21 (20/78) | 72 (13/185) |
| Sheep | - | - | 464 (348/465) |
| ty at 22°C (in mPa) | 40% HCT | 50% HCT | 60% HCT |
| Man | 3 (1/8) | 21 (10/21) | 21 (12/39) |
| Horse | 27 (9/46) | 20 (10/30) | 60 (20/185) |
| Sheep | - | - | 214 (213/215) |
| ty at 37°C (in mPa) | 40% HCT | 50% HCT | 60% HCT |
| Man | - | 9 (4/9) | 9 (4/18) |
| Horse | - | 10 (9/18) | 54 (4/189) |
| Sheep | - | - | 97 (31/99) |
Table 1: Yield points (ty) of human, equine and ovine blood at 10 rad s-1. Increasing strains of 0.001–10 Pa were applied by a logarithmic shear stress ramp. Data are presented as median values and quartiles in parentheses.
| G´9rad/s at 7°C in mPa | 40% HCT | 50% HCT | 60% HCT |
|---|---|---|---|
| Man | 80 (68/117) | 262 (253/289) | 441 (410/534) |
| Horse | 176 (145/221) | 260 (238/281) | 340 (311/415) |
| Sheep | 20 (8/49) | 64 (49/78) | 146 (123/238) |
| G´9rad/s at 22°C in mPa | 40% HCT | 50% HCT | 60% HCT |
| Man | 31 (23/51) | 132 (116/151) | 259 (225/289) |
| Horse | 92 (70/109) | 132 (130/139) | 179 (161/216) |
| Sheep | 10 (5/11) | 23 (17/35) | 92 (79/107) |
| G´9rad/s at 37°C in mPa | 40% HCT | 50% HCT | 60% HCT |
| Man | 19 (13/28) | 77 (69/92) | 171 (159/181) |
| Horse | 56 (31/81) | 77 (66/80) | 119 (114/135) |
| Sheep | 3 (2/7) | 21 (11/25) | 58 (50/66) |
Table 2: Storage modulus at 9.38 rad s-1 in human, equine and ovine blood samples (CSS mode, 5-10 mPa shear stresses). Data are expressed as median and quartiles in parentheses.
Figure 1: Storage modulus (G´) increases with the cellular fraction (hematocrit; HCT) in each species (blue=man; green=horse; yellow=sheep). The increase of G´ with the HCT is blunted in the horse compared to man at each temperature. Note the different scaling on the y-axis to show the differences between the species. Boxes show median and quartiles.
Discussion
The present investigation shows that the elastic behavior of whole blood from mammalian species can be obtained by small amplitude oscillation, but it requires the detection of low torques. To study the influence of functional RBC properties, we tested blood from three species: the horse representing the species with highest RBC aggregability of all, followed by man with intermediate, and sheep with nearly immeasurable RBC aggregability [16]. All individuals were adult, clinically healthy, and showed a regular hematological profile. Measures were performed at the physiological body temperature of man, and at two lower temperatures. The lower temperatures were chosen because animal and human blood is used in the forensic discipline of bloodstain pattern analysis (BPA). Training of BPA analysts and the re-enactment of criminal cases needs blood that is often stored in the fridge. Our data demonstrate the importance of careful temperature adjustment of blood bags prior to their use. Also, the cooling of blood during the flight of shed droplets may be important for the stain during wintertime. The samples were adjusted to predetermined HCT values to compare between the species. By this approach, we demonstrated that the shear moduli increased with the HCT and decreased with the temperature while the gradient of this increase was species-specific. Since blood is thixotropic, time dependent phenomena must be considered especially at low shear. Such an effect could have been present at 40% HCT and 37°C because of RBC sedimentation in the double-gap cylinder system. Since we started our amplitude sweep tests with 1 mPa, phase separation may have occurred during the measurement resulting in sloping G´-vales at the strain increments. In fact, yield points became easier detectable at higher HCT and lower temperature.
The comparative approach is still an important step to understand the relationship between the quality of blood components and rheological outcomes. As expected from the differences in RBC aggregability among horse, man, and sheep RBCs, the shear moduli varied, being highest in the horse. In fact, it was difficult to measure ovine blood, which likely is the result of the minute RBC aggregability of this species. Since blood is a viscoelastic fluid, G´-values were always below loss modulus (G´´)-values. A percolated structure in normal plasma could only be achieved at very high and thus unphysiologic HCT values that are normally present only in the spleen. In such circumstances blood behaved jellylike, a property that might facilitate storage of RBCs within the fibrous structures of this organ.
At a frequency analog to the physiologic heart rate, G´ was higher in horse blood than in human blood at the physiological HCT (40%), but lower in horse compared to man when the HCT was raised (60%) (Figure 1). This implicates that the gradient of the G´-increase in relation to the cellular fraction in blood was lower in the horse. This could be of physiological relevance in this species. Resting horses possess a splenic reservoir for RBCs with a HCT of about 80%. After an exercise-induced contraction, these splenic RBCs are added to the RBCs in the circulation thereby increasing HCT to maximally 65% [35]. The extra number of circulating RBCs effectively increases the blood oxygen store and the oxygen carrying capacity. However, in parallel, the blood viscosity and the elasticity of the blood suspension is elevated, as well. On the other hand, the low dependency of G´ on the HCT value could be a benefit during exercise.
Sheep is a totally different species in regard to its RBC properties. Ovine RBCs are smaller than those of man and horse [36] and have a reduced deformability if exposed to shear stress [37]. Based on their minute RBC aggregability [16], RBCs are singularly suspended in the plasma volume. Flow curves show the low shear thinning of ovine blood. Sheep blood also shows low thixotropy, which can be referred to its low RBC sedimentation. An interesting finding of sheep blood concerns the yield point that could not be measured at physiological HCT, possibly due to the low cohesive forces between RBCs. However, at a HCT of 60% the yield point was higher than in man and horse. The physiological HCT of ovine blood ranges between 30 and 38% [36]. A release of splenic RBCs by a sympathetic stimulus is insignificant in sheep due to the diverse composition and function of the spleen. Sheep do not exhibit high HCT values regularly. But if they do so, the yield stress of blood is higher than in man and horse. The clinical relevance of this finding has to be explored.
It must be kept in mind that blood flow in the vasculature is nonlinear due to its pulsation with the cardiac rhythm [38]. In addition, pulse propagation along the wall of elastic arteries further complicates the flow profile [39]. At physiological circumstances, blood can be subjected to large amplitudes due to these combined axial and transversal oscillations. Recently, strain and stress normalized Lissajous-Bowditch plots were performed to quantify the nonlinear viscoelastic property of blood [40]. The authors found out that the viscous behaviour was dominant over the elastic behaviour at strain amplitudes above 50. This result supports our finding pertaining to the elastic character of blood, which we also measured at low strain only. Although the informative value of tests under linear mode is limited to cover the full range of oscillations that occur when blood flows through the vascular compartments, shear moduli under low amplitude can help to understand fluid vesicle or micellar systems at similar conditions [41]. Such experimental values may also be helpful in validating numerical simulations. It must also be considered that intravascular shear rates are not fixed throughout the body, but vary dynamically [42]. A decrease in blood flow either generalized due to blood loss or due to vascular dysfunction such as in septicaemia, or more localized due to vascular occlusion following intima hyperplasia or thromboembolic events can lower the strain amplitudes in arterioles relevantly. Since species differences could be pointed out, our next step will be to investigate if the elastic behaviour of blood can be used predictively. For instance, in hyperfibrinogenemia or in diseases associated with a change in the intrinsic mechanical properties of blood cells, the elastic behaviour of blood could vary.
Conclusion
In conclusion, blood is a fragile suspension that shows its best stability around the resting heart rate of the species. At frequencies above 15 rad s-1 and below 3 rad s-1, G´-values were significantly sloping, showed an irregular sequence, or were even absent. We assume that the weak forces within a blood sample are disrupted at high frequencies, while at low frequencies; sedimentation of RBCs in the rheometer gap may result in phase separation and shear banding. Although it is logical that suspension stability will increase with the increase of the volume fraction of cellular elements in a composite fluid, we show on our HCT standardized blood samples that G´-values and yield stresses are higher if RBCs have the ability and chance to form aggregates.
References
- Waugh RE, Hochmuth RM (2000) The biomedical engineering handbook: mechanics and deformability of hematocytes. CRC Press, pp 474-486.
- Branemark PI, Bagge U (1977) Intravascular rheology of erythrocytes in man. Blood Cells 3: 11-24.
- Fedosov DA, Peltomäki M, Gompper G (2014) Deformation and dynamics of red blood cells in flow through cylindrical microchannels. Soft Matter 10: 4258-4267.
- Gaehtgens P, Schmidt-Schönbein H (1982) Mechanism of dynamic flow adaptation of mammalian erythrocytes. Naturwissenschaften 69: 294-296.
- Fischer TM (2004) Shape memory of human red blood cells. Biophys J 86: 3304-3313.
- Park YK, Best CA, Auth T, Gov NS, Safran SA, et al. (2010) Metabolic remodeling of the human red blood cell membrane. PNAS 107: 1289-1294.
- Gaehtgens P (1981) Microrheology of blood in capillaries. Drug Research 31: 1995-1998.
- Basu H, Dharmadhikari AK, Dharmadhikari JA, Sharma S, Mathur D (2011) Tank threading of optically trapped red blood cells in shear flow. Biophys J 101: 1604-1612.
- Dupire J, Socol M, Viallat A (2012) Full dynamics of a red blood cell in shear flow. Proc Natl Acad Sci 109: 20808-20813.
- Fischer TM (2007) Tank thread frequency of the red cell membrane: Dependence on the viscosity of the suspending medium. Biophys J 93: 2553-2561.
- Tran-Son-Tray R (2006) A study of the tank thread motion of red blood cells in shear flow. PhD thesis Washington University, USA
- Barshtein G, Wajnblum D, Yedgar S (2000) Kinetics of linear Rouleaux formation studied by visual monitoring of red cell dynamic organisation. Biophys J 78: 2470-2474.
- Brust M, Aouane O, Thiebaud M, Flormann, Verdier C, et al. (2014) The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Scientific Reports 4: 4348
- Cokelet GR, Meiselman HJ (2007) Handbook of hemorheology and hemodynamics. Macro- and micro-rheological properties of blood. IOS Press Washington DC, pp: 45-71.
- Johnson PC, Bishop JJ, Popel S, Intaglietta M (1999) Effects of red cell aggregation on the venous microcirculation. Biorheology 36: 457-460.
- Windberger U, Bartholovitsch A, Plasenzotti R, Korak HJ, Heinze G (2003) Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: Reference values and comparison of data. Exp Physiol 88: 431-440.
- Windberger U, Baskurt OK (2007) Handbook of hemorheology and hemodynamics. Comparative hemorheology. IOS Press Washington DC, pp 267-285.
- Lowe GDO (1998) Clinical blood rheology, Boca Raton Florida. CRC Press, pp: 1-2.
- Cabel M, Meiselman HJ, Popel AS, Johnson PC (1997) Contribution of red blood cell aggregation to venous vascular resistance in skeletal muscle. Am J Physiol 272: H1020-H1032.
- Toth K, Kesmarky G, Alexy T (2007) Handbook of hemorheology and hemodynamics. Clinical significance of hemorheologic alterations. IOS Press Washington DC, pp: 392-432.
- Bishop JJ, Nance PR, Popel AS, Intaglietta M, Johnson PC (2001) Effect of erythrocyte aggregation on velocity profiles in venules. Am J Physiol Heart Circ Physiol 280: H222-H236.
- Soutani M, Suzuki Y, Tateishi N, Maeda N (1995) Quantificative evaluation of flow dynamics of erythrocytes in microvessels: influence of erythrocyte aggregation. Am J Physiol Heart Circ Physiol 268: H1959-H1965.
- Charansonney O, Mouren S, Dufaux J, Duvelleroy M, Vicaut E (1993) Red blood cell aggregation and blood viscosity in an isolated heart preparation. Biorheology 30: 75-84.
- Pantos J, Efstathopoulos E, Katritsis G (2007) Vascular wall shear stress in clinical practice. Curr Vasc Pharmacol 5: 113-119.
- Kim S, Popel AS, Intaglietta M, Johnson PC (2005) Aggregate formation of erythrocytes in postcapillary venules. Am J Physiol Heart Circ Physiol 288: 584-590.
- Bishop JJ, Popel AS, Intaglietta M, Johnson PC (2001) Effects of erythrocyte aggregation and venous network geometry on red blood cell axial migration. Am J Physiol Heart Circ Physiol 281: H939-H950.
- Moncada S, Palmer RM, Higgs EA (1981) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43: 109-142.
- Malek K, Izumo S (1992) Physiological fluid shear stress causes down-regulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol 263: C389-C396.
- Ong PK, Jain S and Kim S (2012) Spatio-temporal variations in cell-free layer formation near bifurcations of small arteries. Microvasc Res 83: 118-125.
- Hightower CM, Vazquez BY, Park SW, et al. (2011) Integration of cardiovascular regulation by the blood/endothelium cell free layer. Wiley Interdiscip Rev Syst Biol Med 3: 458-470.
- Yalcin O, Aydin F, Ulker P, et al. (2006) Effects of red blood cell aggregation on myocardial haematocrit gradient using two approaches to increase aggregation. Am J Physiol Heart Circ Physiol 290: H765-H771.
- Tomaliuolo G (2014) Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 8: 051501.
- Baskurt OK, Meiselman H (2003) Blood rheology and hemodynamics. Semin Thromb Hemost 29: 435-450.
- Tomaliuolo G, Carciati A, Caserta S, Guido S (2015) Blood linear viscoelasticity by small amplitude oscillatory flow. Rheologica Acta 5: 1-11.
- Persson SGB (1983) Equine exercise physiology: The significance of haematological data in the evaluation of soundness and fitness in the horse. Snow & Persson & Rose Granta Editions Cambridge UK.
- Kraft W, Duerr UM (2005) Klinische Labordiagnostik in der Tiermedizin. Schattauer Stuttgart New York.
- Plasenzotti R, Stoiber B, Posch M, Windberger U (2004) Red blood cell deformability and aggregation behaviour in different animal species. Clin Hemorheol Microcirc 31: 105-111.
- Sousa PC, Pinho FT, Alves MA, Oliveira MSN (2016) A review of hemorheology: Measuring techniques and recent advances. Korea-Australia Rheology Journal 28: 1-22.
- Herrera-Valencia EE, Sanchez-Villavicencio ML, Calderas F, Perez-Camacho M, Medina-Torres L (2016) Simultaneous pulsatile flow and oscillating wall of a non-Newtonian liquid. Korea-Australia Rheology Journal 28: 281-300.
- Sousa PC, Carneiro J, Pinho FT, Oliveira MSN, Alves MA (2013) Shear viscosity and non-linear behavior of whole blood under large amplitude oscillatory shear. Biorheology 50: 269-282.
- Miller E, Rothstein JP (2007) Transient evolution of shear banding wormlike micellar systems. J Non-Newtonian Fluid Mech 143: 22-37.
- Pries AR, Secomb TW (2003) Rheology of the microcirculation. Clin Hemorheol Microcirc 29: 143-148.
Citation: Windberger U, Stoiber B, Pöschl C, van den Hoven R (2016) A Comparative Approach to Measure Elasticity of Whole Blood by Small Amplitude Oscillation. Rheol: open access 1: 103.
Copyright: © 2016 Windberger U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4131
- [From(publication date): 0-2017 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 3208
- PDF downloads: 923