A Commentary on Drug-Drug Interaction between Tacrolimus and Caspofungin in Chinese Kidney Transplant Patients with Different CYP3A5 Genotypes
Received: 16-Aug-2024 / Manuscript No. JIDT-24-145543 / Editor assigned: 19-Aug-2024 / PreQC No. JIDT-24-145543 (PQ) / Reviewed: 02-Sep-2024 / QC No. JIDT-24-145543 (QC) / Revised: 09-Sep-2024 / Manuscript No. JIDT-24-145543 (R) / Published Date: 17-Sep-2024 DOI: 10.4172/2332-0877.24.S8.005
Abstract
Tacrolimus is a first-line immunosuppressant to reduce the incidence of graft rejection after kidney transplantation, which may potentially interact with various drugs, resulting in altered blood concentrations. Caspofungin, an echinocandin antifungal agent, is frequently co-administered with tacrolimus to treat fungal infections in kidney transplant patients. The previously published article titled "Drug-drug interaction between tacrolimus and caspofungin in Chinese kidney transplant patients with different CYP3A5 genotypes" provides a concise review, focusing specifically on CYP3A5 genotypes affect the interaction between tacrolimus and caspofungin. A thorough understanding of drug interactions is critical for optimizing immunosuppressive therapy, reducing adverse drug reactions, and ultimately achieving more effective individualized dosing for kidney transplant patients.
Keywords: Tacrolimus; Caspofungin; CYP3A5; Drug-drug interactions; Kidney transplantation
Introduction
As a first-line immunosuppressant used in patients after kidney transplantation surgery, the effectiveness and safety of tacrolimus in preventing graft rejection largely depend on maintaining stable blood concentrations [1]. However, tacrolimus metabolism is influenced by the activity of the cytochrome P450 3A (CYP3A) enzymes, which in East Asian populations mainly refers to changes in enzyme activity affected by CYP3A5 gene polymorphism. Specifically, CYP3A5 expressers (CYP3A5*1/*1, *1/*3 have higher enzyme activity, while non-expressers (CYP3A5*3/*3) have lower enzyme activity. The differences in CYP3A5 enzyme activity lead to variations in the metabolism of tacrolimus, resulting in individual differences in blood concentrations and therapeutic effect [2]. Invasive fungal infections are among the leading causes of morbidity and mortality in solid organ transplant recipients. Caspofungin, the first echinocandin antifungal drug with definite activity against azole-resistant candida species, is widely recommended for the treatment of invasive candidiasis [3].
Previous reports have suggested that caspofungin may alter the blood concentration of tacrolimus, potentially affecting its efficacy and safety however, real-world evidence remains very limited [4].
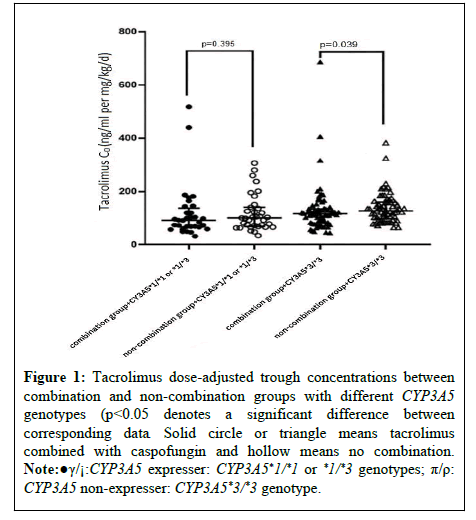
In this study, 476 eligible participants were included from an initial pool of 1524 post-kidney transplant patients treated with tacrolimus, consisting of 171 patients who were co-administered caspofungin and 305 patients who were not. Overall, the combined use of caspofungin was associated with a reduction in tacrolimus blood concentrations, although the difference between the two groups did not reach statistical significance. This study further conducted a subgroup analysis, focusing specifically on the drug-drug interaction between tacrolimus and caspofungin under different CYP3A5 genetic polymorphisms. As shown in Figure 1, in CYP3A5 non-expressers, the interaction led to a significant decrease in tacrolimus blood concentration levels (116.21 (81.11, 134.00) ng/mL/(mg/kg/day) vs. 126.50 (101.34, 158.18) ng/ mL/(mg/kg/day), p=0.039). However, no significant difference was observed among CYP3A5 expressers (p=0.359).
The data in Table 1 showed that in patients co-administered with caspofungin, CYP3A5 non-expressers would require an approximately 11.11% increase in the daily dose of tacrolimus to maintain blood concentrations comparable to the non-combination group. In contrast, for CYP3A5 expressers, the daily dose of tacrolimus needed to be increased by only 4.76% when combined with caspofungin compared to the control group.
| Patients | Tacrolimus daily dose (mg/kg/day) | p-value | ||
|---|---|---|---|---|
| Non-combination group | Combination group | %Change in dose | ||
| Total (n=200) | 0.096 (0.065, 0.113) | 0.100 (0.070, 0.119) | 4.17 | 0.544 |
| CYP3A5*1/*1 or *1/*3 (n=67) | 0.105 (0.071, 0.123) | 0.110 (0.081, 0.123) | 4.76 | 0.851 |
| CYP3A5*3/*3 (n=133) | 0.090 (0.063, 0.108) | 0.100 (0.066, 0.111) | 11.11 | 0.449 |
Table 1: Effect of caspofungin on the required daily dose of tacrolimus under different CYP3A5 genotypes.
This finding is of great significance for guiding clinical dose adjustments and Therapeutic Drug Monitoring (TDM) for tacrolimus. Previous studies have shown that caspofungin can significantly reduce tacrolimus blood concentrations [5], likely due to its mild induction of the CYP3A enzyme system, which accelerates the metabolism of tacrolimus in the intestines and liver, thereby lowering its blood levels.
Although this inductive effect is relatively weak, the effect of caspofungin should not be ignored in kidney transplant patients who require long-term treatment of tacrolimus. In CYP3A5 non-expressers, who have lower enzyme activity and slower tacrolimus metabolism, the inductive effect of caspofungin on the enzyme is more evident. This is likely due to the accumulation of tacrolimus, resulting in more significant changes in its blood concentration. However, there have been conflicting results. A study by Nishimoto et al., on the interaction between caspofungin and tacrolimus during allogeneic hematopoietic stem cell transplantation did not yield positive results [6]. Saner et al., concluded that the combination of caspofungin and tacrolimus is safe in liver transplant patients [7].
Although previous studies offer preliminary insights into the interaction between tacrolimus and caspofungin, several issues remain unresolved. Firstly, genetic variants influence the metabolism of tacrolimus and drug-drug interactions, while the underlying mechanisms are still poorly understood. Secondly, the extent of the drug-drug interactions may vary depending on the doses of tacrolimus or caspofungin, necessitating further research under different dosing conditions to elucidate these interactions more clearly. Thirdly, in real clinical settings, patients may be administered multiple medications concurrently, which could make the situation more complicated. Therefore, personalized adjustments and monitoring based on individual patient circumstances are essential in clinical practice.
Based on previous findings, we are developing a personalized dosing model for tacrolimus in post-kidney transplant patients. This model will integrate genetic factors, drug interactions and other clinically relevant variables to create an initial framework. We will then validate this model through a Randomized Controlled Trial (RCT) to evaluate its effectiveness and applicability. This approach will enable clinicians to better predict and manage potential drug interactions, ultimately enhancing treatment outcomes and providing a foundation for personalized medication strategies in post-kidney transplant patients who are on multiple medications treatment.
References
- Coste G, Robin F, Chemouny J, Tron C, Le Priol J, et al. (2022) Drug transporters are implicated in the diffusion of tacrolimus into the T lymphocyte in kidney and liver transplant recipients: Genetic, mRNA, protein expression, and functionality. Drug Metab Pharmacokinet 47:100473.
[Crossref] [Google Scholar] [PubMed]
- Sallustio BC, Noll BD, Hu R, Barratt DT, Tuke J, et al. (2021) Tacrolimus dose, blood concentrations and acute nephrotoxicity, but not CYP3A5/ABCB1 genetics, are associated with allograft tacrolimus concentrations in renal transplant recipients. Br J Clin Pharmacol 87:3901-3909.
[Crossref] [Google Scholar] [PubMed]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, et al. (2016) Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 62:e1-e50.
[Crossref] [Google Scholar] [PubMed]
- Sable CA, Nguyen BY, Chodakewitz JA, DiNubile MJ (2002) Safety and tolerability of caspofungin acetate in the treatment of fungal infections. Transpl Infect Dis 4:25-30.
[Crossref] [Google Scholar] [PubMed]
- Cheng S, Tang M, Du J, Yin T (2022) Effects of antifungal drugs on the plasma concentrations and dosage of tacrolimus in kidney transplant patients. Eur J Hosp Pharm 29:202-206.
[Crossref] [Google Scholar] [PubMed]
- Nishimoto M, Koh H, Tokuwame A, Makuuchi Y, Kuno M, et al. (2017) Drug interactions and safety profiles with concomitant use of caspofungin and calcineurin inhibitors in allogeneic haematopoietic cell transplantation. Br J Clin Pharmacol 83:2000-2007.
[Crossref] [Google Scholar] [PubMed]
- Saner F, Gensicke J, Rath P, Fruhauf N, Gu Y, et al. (2006) Safety profile of concomitant use of caspofungin and cyclosporine or tacrolimus in liver transplant patients. Infection. 34:328-332.
[Crossref] [Google Scholar] [PubMed]
Citation: Zong H, Li Y (2024) A Commentary on Drug-Drug Interaction between Tacrolimus and Caspofungin in Chinese Kidney Transplant Patients with Different CYP3A5 Genotypes. J Infect Dis Ther 12:606. DOI: 10.4172/2332-0877.24.S8.005
Copyright: © 2024 Zong H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 116
- [From(publication date): 0-2024 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 87
- PDF downloads: 29