A Combined Approach of CEUS and DEMR in the Diagnosis of Parathyroid Hyperparathyroidism
Received: 04-May-2018 / Accepted Date: 24-May-2018 / Published Date: 30-May-2018 DOI: 10.4172/2167-7964.1000297
Abstract
Background: Ultrasound detection of parathyroid hyperparathyroidism solely depends upon radiologist experience in all modalities. Large nodule more than 5 mm can be detected confidently in ultrasound alone with proper history. However nodule<2 mm, negative ultrasound, not traced nuclear medicine study, further patients have persistent primary hyperparathyroidism symptoms and biochemical parameters increased is challenging task. This small nodule establishing diagnosis with appropriate surgery treatment planning in symptomatic patients from minimal invasive surgery to total neck exploration alarms endocrine surgeons. Our study emphasis on value of advanced MR and CEUS imaging protocol in detecting even small nodules as compared to conventional CT and MR wherein sensitivity and specificity is low and moreover CT is radiation modality.
Objectives: This study directly compared diagnostic value of dynamic enhanced DEMR and 4D CT application for preoperative detection of solitary parathyroid nodule.
Patients and methods: 31 patients selected as sample volume. 18 positive patients detected incidental parathyroid nodule by USG with biochemical parameters were grouped for further evaluation. 3 patients showed negative ultrasound, but with increased lab value subjected to SPECT and lesion localized and grouped along with positive cases. 10 patients were initial suspected clinically but normal lab value and negative ultrasound set as control group in our study. Each patient provided written informed consent to participate in this study. All positive patients subjected to CEUS and 4D MR and within 2 weeks surgery planning done accordingly and specimen cytology results compared for positive cases as gold standard. Results: Surgery specimen revealed 16 solitary parathyroid adenomas, 3 parathyroid carcinoma, and 2 patients with atypical parathyroid lesion like cyst with no complication. These results are correlated with CEUS and 4D MR results. No evidence of multiple lesions/diffuse involvement noted in our study and these results are correlated with HPE. The diagnostic sensitivities for detection of parathyroid adenomas of 66% sensitivity and 94% specificity, PPV 66%, NPV 94% and accuracy 80% (n=21) by CEUS and DEMR.
Conclusion: This study demonstrates that CEUS/DEMR is more sensitive in preoperative localization of parathyroid adenomas versus parathyroid carcinoma and reduces morbidity by selective minimal invasive planning.
Keywords: Primary hyperparathyroidism; CEUS; DEMR; Histopathology; Parathyroid incidentaloma
Abbreviation
DEMR: Dynamic Enhanced Magnetic Resonance; CEUS: Contrast Enhanced Ultrasound; PTH: Parathyroid Hyperparathyroidism
Introduction
Primary hyperparathyroidism (PHPT) single entity is caused by multiple pathology. Commonest cause for PHPT adenoma, followed by hyperplasia with multioglandular involvement and sometimes carcinoma presented as solitary lesions or atypical cysts difficult in establishing diagnosis. Incidence rate, PHPT as from parathyroid adenoma (80%), Parathyroid hyperplasia (10%-15%), Multiple parathyroid adenomas (4%), Parathyroid carcinoma (1- 5%). Parathyroid glands embryological origin is complex nature, deriving from pharyngeal pouch and usually located behind the two thyroid lobe superior aspects (upper parathyroid glands) and usually 3 to 4 in numbers and two localized behind the inferior third of thyroid gland. The familial forms and associated PTA conditions include: Multiple Endocrine Neoplasia (MEN 1 and MEN 2) and isolated familial hyperparathyroidism associated with jaw tumors (HPT-jaw tumor syndrome). In MEN I the endocrine glands most affected are parathyroid glands, pancreas, and pituitary glands [1-3].
The spectrum of symptoms that may represent the clinical manifestation of PTI and PHPT is very wide. Among Hyperparathyroidism subtypes, primary (PHPT) contributes significantly, results from internal or intrinsic parathyroid hormone changes rather than external or extrinsic calcium homeostasis affecting etiology as seen in Secondary Hyperparathyroidism (SHPT). Parathyroid Adenomas (PTA), the most common cause of primary hyperparathyroidism, are benign tumours which autonomously produce and secrete parathyroid hormone. Primary hyperparathyroidism not uncommon disease as compared to most common endocrine pathology like diabetes and thyroid abnormality with the highest incidence seen in postmenopausal women [4-6].
Histologically, adenoma is most common in PHPT present in two forms, micro-adenoma, typically encapsulated with indistinct fibrous capsule as compared to large adenomas with well-defined fibrous capsule. Adenoma moreover seen in follicles of sheets is most commonly around the blood vessels. Whereas Carcinoma is eccentric located and malignant fibrous proliferation and surrounding structures margins are ill defined.
By non-invasive diagnostic techniques, ultrasound standout as first modality of choice. Detection rate and Sensitivity in diagnosing adenoma is related to size of the lesion.
Larger the adenoma or lesion size in parathyroid, easy to establish the diagnosis. Smaller the lesions, more becomes operator dependent in picking up lesion and ruling out false positive diagnosis. Parathyroid volume calculation is helpful in delineating other pathologies like hyperplasia, multiple locations and multiple lesions diagnostic size criteria to be considered [7-9]. Up to 5% of parathyroid adenomas can occur in ectopic locations. Foci of cystic change are particularly common in large adenomas. Ectopic located parathyroid are difficult in establishing diagnosis by Sonology and role of radiation modalities like CT and Sestamibi Scan serves as problem solving tool. Majority of incidental solitary nodular parathyroid lesion are situated in juxtra thyroid location in parathyroid gland. Common ectopic location, where CT helps in trachea-esophageal groove/paraoesophageal or mediastinum. Localization of the parathyroid glands can be really variable, especially the site of the lower glands, due to the longer pathway and difficult migration process. Common ectopic locations include mediastinum, retropharyngeal, carotid sheath, intrathyroidal, thyrothymic ligament, Superior thyroid poles, Trachea oesophageal grooves, Retro oesophageal space, and carotid sheath. Most people have four parathyroid glands, although supernumerary glands have been found in 13% of the population, most commonly in the thymus.
Atypical adenomas of the parathyroid glands represent a controversial entity. These tumors have some of the features of parathyroid carcinomas, but they lack the unequivocal evidence of invasive growth (peritumoral vascular invasion, perineural invasion, or invasion of the adjacent soft tissues or thyroid.
Parathyroid carcinoma is an uncommon tumor, which most often appears as a large mass that is densely adherent to the surrounding soft tissues or the thyroid gland. The diagnosis of malignancy should be restricted to those cases that show invasion of the adjacent soft tissues or thyroid gland, blood vessels, or perineural spaces, and to those tumors with documented metastases [6-8]. Fibrous band formation within the tumor is common, but by itself, this feature is insufficient for the diagnosis of malignancy [7-9].
The term parathyroid incidentaloma (PTI) was newly coined entity, where usually seen in symptomatic patient with Primary or secondary hyperparathyroidism, high resolution ultrasound depicting solitary nodule in parathyroid region. Otherwise PTI can be seen incidental finding sometimes, usually less than 10 mm solitary nodules in asymptomatic patients. Most common differential to be considered in small PTI<10 mm can be multinodular goitre or perithyroid nodas misleading as parathyroid nodule or vice versa. Role of Sonologist experience plays a dominant role in these conditions.
Parathyroid cysts are grouped under atypical lesions in few studies, as cysts could be functioning or non-functioning nature. Functioning cyst diagnosis can only be established by Serum Calcium and Parathormone level along with Clinical symptoms. By imaging, functional or non-functional status of the cyst difficult to comment upon, even in dynamic enhanced ultrasound or CT/MR. However large parathyroid cysts by follow up study increasing size could raise the suspicion of complexity and functioning nature along with other pressure effects due to size like dysphagia and recurrent laryngeal nerve palsy. Sestamibi can yield mixed Sensitivity and Specificity in establishing functioning status [9-11].
Traditionally, ultrasound and Technetium-99 m (99 mTc) Sestamibi scintigraphy have been used as first line tools to localize parathyroid adenomas (PTA). Ultrasound takes advantage of differential echogenicity of PTA compared to thyroid tissue, and scintigraphy takes advantage of physiologically differences in radiopharmaceutical uptake and retention.
In USG, The adenomas are oval or bean-shaped, but larger adenomas can be multilobulated. The vast majority (up to 87%) of adenomas occur as solitary lesions. The average size of a normal parathyroid is 5 × 3 × 1 mm; normal glands weigh between 40 and 50 mg. They are thus infrequently identified at imaging most nodules need to be>1 cm to be confidently seen on ultrasound. Sonologically parathyroid adenoma usually hypoechoic with separating line of adjacent thyroid capsule observed from the gland of thyroid. Smaller size of adenoma, this usual presentation may not be appreciated [12-15].
In some instances Doppler do play role in PTI, adenoma possess characteristic feeding vessel from extra thyroidal branch of inferior thyroidal artery Doppler USG can commonly show a characteristic extrathyroidal feeding vessel. Peripheral vascularity is commonly observed in adenoma conditions as compared to carcinoma where central and irregular color Doppler flow pattern seen. In parathyroid cystic pathology usually CDFI (Color Doppler Flow Imaging) may not be helpful unless there is solid or complexity noted within the cyst [16-19].
By 4D-CT parathyroid adenomas detection rate is very high but at the expense of radiation. Very common presentation by 4D CT diagnosis of adenoma is characteristic low attenuation in non-contrast, significant arterial enhancement in arterial phase.
Polar sign is also observed in enhanced sequences where enlarged feeding artery and draining vein is seen along with early wash out of adenoma. By HU value also 4DCT helps in delineating pathology to extent by increasing HU in 4D phase and ectopic location pickup already been discussed [4-6]. Role of carcinoma and atypical lesion diagnosis is challenging in 4DCT alone, if combined with USG can improve the Sensitivity and Specificity ratio.
Technetium (TC) 99 m Sestamibi (MIBI) commonly used agent works by mitochondrial uptake of 99 m TC Sestamibi and para thyroid cells have a large number of mitochondria.
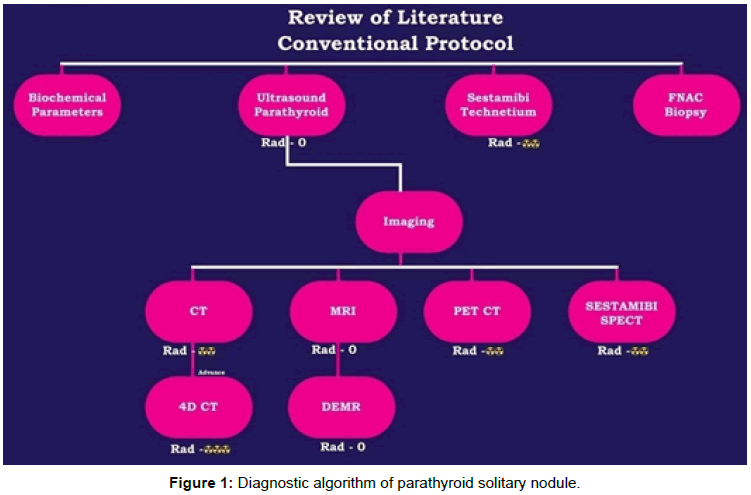
SPECT-CT study is to increase diagnostic accuracy and aid in anatomical localisation. 18F-fluorocholine PET/CT may also have a role but studies showing mixed sensitivity [7,8]. In recent times, the use of labeled amino acid 11C-methionine for the detection of parathyroid adenomas has been proposed. Figure 1 showing diagnostic algorithmic approach for detecting parathyroid nodules.
Several articles have described the high sensitivity of 11C-methionine to localize in the abnormal parathyroid glands [6,7]. In a majority of these studies, 11C-methionine has proved to be superior to 99 mTc-sestamibi or other conventional imaging techniques, with sensitivities and excellent specificity around 85%.
As compared to past decades, rather than complete surgical exploration, Minimal Invasive approach is current trend among endocrine surgeons. But role of imaging and radiologist plays crucial role in diagnostic dilemma and imaging options choice.
As non-radiating, non-invasive is first priority for most endocrine surgeons like ultrasound and dynamic enhanced MR impact is higher. Only in limitations of ultrasound, 4DCT and sestamibi scan diagnosis outweighs radiation effects. If proper diagnostic imaging approach and workup combined with intra operative parathormone level testing can yield good sensitivity/specificity ratio for minimal invasive surgical approach. Intra operative Parathormone level drops<60% upon resection of parathyroid nodule indicates minimally invasive surgical approach is successful and also denotes surgery in right direction and least failure rates. In summation accurate localization imaging protocol selection depends upon clinical symptoms, biochemical parameters and size/complexity of lesions and surrounding structures involvement by using ultrasound. Evaluation is baseline. Other modality role depends upon ultrasound as primary modality directions. Moreover if contrast enhanced ultrasound is combined with baseline ultrasound then other modality role can be minimized, but literature background is lacking in this scenario.
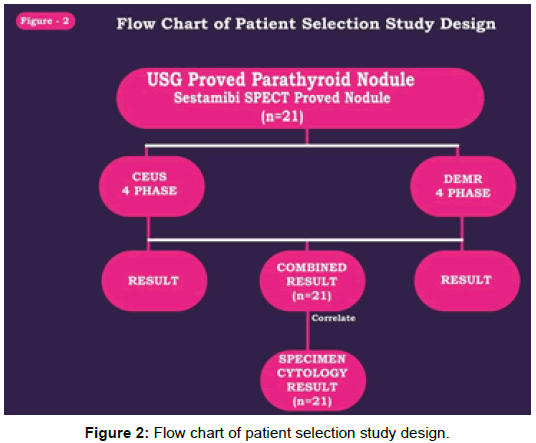
The purpose of the study, non-invasive and non-radiation modality approach to localize the parathyroid pathology with better sensitivity and specificity by combining CEUS and 4D DEMR and thereby helping endocrine surgeon for better surgery planning (Figure 2).
Methodology
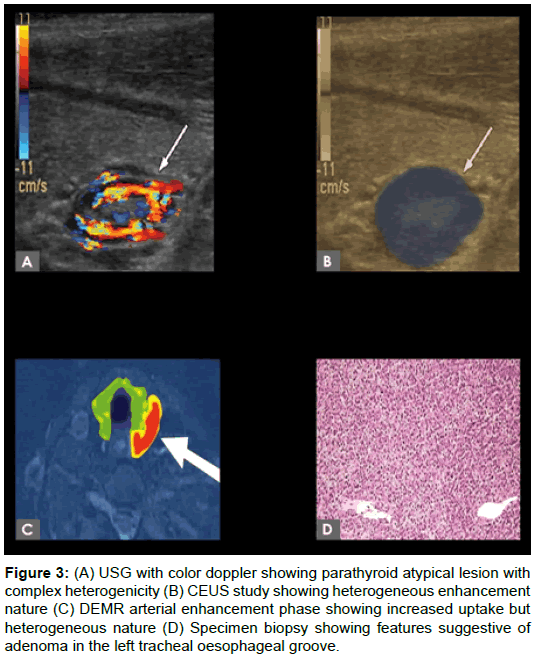
Between August 2016 and December 2017, 31 patients from master health checkup attending our ultrasound clinic were enrolled (M:F=17:14; mean age, 49 ± 10 years; range, 26-70 years). 18 positive patients detected incidental parathyroid nodule by USG with biochemical parameters were grouped for further evaluation. 3 patients showed negative ultrasound, but with increased lab value subjected to SPECT and lesion localized and grouped along with positive cases. 10 patients were initial suspected clinically but normal lab value and negative ultrasound set as control group in our study. Laboratory tests showed raised serum calcium and parathyroid hormone (PTH) levels to on average 3.14-7.45 mmol/L (normal, 2.10-2.55 mmol/L) and 240- 730 μg/L (normal, 8-74 μg/L), respectively. Figure 3 showing patient selection design, following both CEUS and 4D MR done for positive cases. All patients were scheduled to undergo surgical neck exploration in 2 weeks duration.
Figure 3: (A) USG with color doppler showing parathyroid atypical lesion with complex heterogenicity (B) CEUS study showing heterogeneous enhancement nature (C) DEMR arterial enhancement phase showing increased uptake but heterogeneous nature (D) Specimen biopsy showing features suggestive of adenoma in the left tracheal oesophageal groove.
Statistical analysis
The collected data were analysed with IBM.SPSS statistics software 23.0 Version. To describe about the data descriptive statistics frequency analysis, percentage analysis were used. To find the efficacy of the techniques the Receiver Operator Characteristic (ROC) curve analysis was used to find the sensitivity, specificity, PPV and NPV for comparison of CT, MR and combination of both tools with HPE. To find the significance in categorical data Chi-Square test and Fisher’s exact test was used.
Ethical clearance
The present study (project number: VMCH 2382) was approved by our Institutional Ethics Committee. Each patient provided written informed consent to participate in this study.
USG protocol
A routine neck examination done for suspected parathyroid enlargement. All the patients subjected in two planes, bilateral longitudinal and transverse with enface images were included from the carotid arteries to the midline, Upper and lower level from above sterna notch up to supra calvicular fossa level, traced for ectopic pathology if clinical symptomatic. Siemens acuson health care with high frequency probe setup machine deployed for the purpose. The test performed mostly in the supine position, with the patient’s head tilted back. Broadband, high-frequency (8-17 MHz) linear probes are used for the identification of lesions in the parathyroid glands. Probes with the frequency of 5-8 MHz are used in patients with increased neck fat level.
CEUS protocol
Siemens Acuson ultrasound system unit was used for CEUS also along with another Siemens machine for backup. SonoVue (Bracco, Italy) 25 mg of lyophilized powder and 5 ml of 0.9% sodium chloride solution were configured into a suspension, and mixed uniformity. The Patients’ necks were hyperextended to expose the parathyroid area. Scanning was performed by one experienced sonographer, who was asked to evaluate the nodule location, size and Doppler flow signals. Focus was located in the trailing edge of the lesion, and the gain was adjusted to display only the boundaries of the lesion. Then, in real time mode, 2.5 ml of US contrast agent was injected intravenously through the ulnar vein, followed by injection of 5 ml of normal saline flush.
DEMR protocol
We use GE 1.5 T MRI for our parathyroid imaging (ACCURA GE Healthcare, Germany). A combination of 20-element head and neck coil is used for radiofrequency signal reception. Our MR imaging protocol includes axial T2w Fat sat, coronal STIR, coronal T1w pre contrast and 4D dynamic contrast-enhanced (DCE) sequences. 4D-DCE imaging is performed in dynamic fashion using 3D volumetric interpolated examination sequence with the following parameters: TR-4.06 ms, first TE-1.31 ms, second TE-2.54 ms, flip angle-9°, matrix-160 mm, FOV- 200 mm, 60 sections-2 mm thick. A total of 0.1 mmol/kg of gadolinium is injected at 4 ml/sec. To improve inhomogeneity of fat suppression in the neck, we used the fat suppression technique. This coverage is adequate to encompass from the inferior mandibular rim to the carina in most patients. Large field-of-view required in parathyroid imaging.
Image Analysis and Interpretation
CEUS (Contrast enhanced ultrasound) analysis
Real‑time CEUS of parathyroid nodules on average persist 30- 45 seconds. Adenomas are much shorter (10-15 sec) as compared to atypical and carcinoma. The contrast‑enhancement patterns of the lesions were categorized according to CEUS grades into 4 subtypes. Category 1-peak enhancement (Average time contrast retained in nodule from wash in subtracting with wash out time calculated). Category 2-Time to Peak (Average time contrast enhancement at its peak in the nodule). Category3-Wash in (Time taken for contrast to reach the nodule after injection) Wash out (Time taken for contrast to wash out from nodule). This 4 categories result are compared and correlated with DEMR by ROC characteristics curve analysis.
DEMR (Dynamic Enhanced MR) analysis
For pre-operative localization, T2w fat-sat and 4D dynamic series are most scrutinized. The majority of Para thyroid nodules are T2 hyperintense and demonstrated early arterial enhancement. Using coregistered images, we placed ROIs over parathyroid adenoma to obtain peak enhancement, time-to-peak, wash-in, and washout in each (again 4 subtypes like CEUS). Results are calculated and with CEUS compared and correlated. Receiver operating characteristic analysis was performed to determine the optimal parameters for determination of parathyroid adenomas versus other nodules.
Results
For each patient, abnormal parathyroid tissue localized at imaging which was associated with a surgically determined correct position and nature of the pathology was considered a true-positive finding. Imaging of localized abnormalities without surgically detected parathyroid focal pathology was considered false-positive findings. SPECT CT done only in 4 patients was negative ultrasound reported.
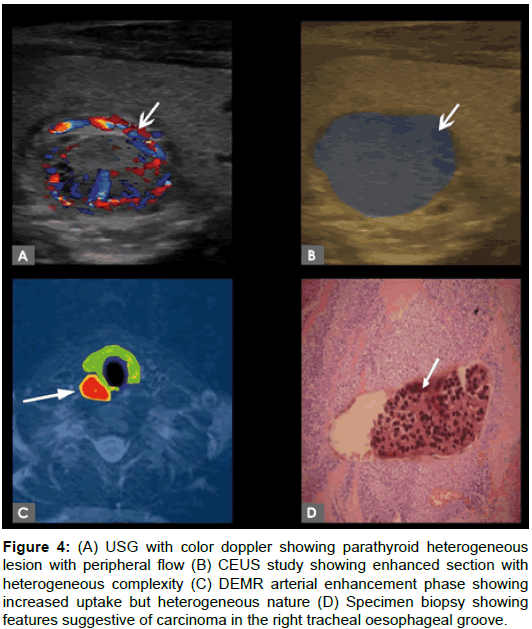
Histopathological results of the minimal invasive parathyroidectomy samples were obtained. Specimen revealed (n=16) solitary parathyroid adenomas, (n=3) parathyroid carcinoma, (n=2) patient with atypical parathyroid lesion like cyst with no complication. No evidence of multiple lesions/diffuse involvement noted in specimen study. Figure 4 showing sensitivity ratio and ROC curve confidence interval ratio. By combining methodology CEUS and DEMR, confidence interval increased as compared with Surgical Histopathology as gold standard.
Figure 4: (A) USG with color doppler showing parathyroid heterogeneous lesion with peripheral flow (B) CEUS study showing enhanced section with heterogeneous complexity (C) DEMR arterial enhancement phase showing increased uptake but heterogeneous nature (D) Specimen biopsy showing features suggestive of carcinoma in the right tracheal oesophageal groove.
Among the 31 patients, mean average age is 49 years from master health checkup attending our ultrasound clinic were enrolled (M:F=17:14; mean age, 49 ± 10 years; range, 26-70 years.
Four category of contrast enhancement, studied in Contrast enhanced ultrasound and Dynamic enhanced MR. Table 1, showing Peak enhancement phase by cross tabulation and chi square test comparison with gold standard biopsy correlation.
A
| DEMR | |||||
|---|---|---|---|---|---|
| Adenoma | Atypical | Carcinoma | Total | ||
| CEUS Adenoma | Number | 11 | 0 | 0 | 11 |
| % | 68.80% | 0.00% | 0.00% | 68.80% | |
| CEUS Atypical | Number | 0 | 2 | 1 | 3 |
| % | 0.00% | 12.5% | 6.3% | 18.8% | |
| CEUS Carcinoma | Number | 1 | 0 | 1 | 2 |
| % | 6.3% | 0.00% | 6.3% | 12.5% | |
| Total | Number | 12 | 2 | 2 | 16 |
| % | 75.0% | 12.5% | 12.5% | 100% | |
B
| Category | Value | df | Asymp-Sig (12- sided) P-Value |
|---|---|---|---|
| Pearson Chi-Square | 16.667 | 4 | 0.002 |
| Likelihood ratio | 16.948 | 3.8 | 0.0019 |
| No. of valid cases | 16 | - | |
Table 1: (A, B) Showing cross tab value of CEUS and DEMR with Chi-Square test (A) Peak enhancement phase (B) Chi-Square test of significance for peak enhancement.
Average time contrast retained in nodule from wash in subtracting with wash out time calculated. There is strong correlation in detecting adenoma by CEUS and Dynamic MRI, around 68.8% for peak enhancement phase. There is weak correlation in detecting atypical lesions and carcinoma by CEUS and DEMR around 12.5% and 6.3%. Overall Chi Square test significance is .002.
Table 2 is showing time to peak enhancement phase by cross tabulation and chi square test comparison with gold standard biopsy correlation. Average time contrast enhancement at its peak in the nodule. There is strong correlation in detecting adenoma by CEUS and Dynamic MRI, around 75.0% for Time to peak enhancement phase. There is minimal to weak correlation in detecting atypical lesions and carcinoma by CEUS and DEMR around 5.0% and 20.0%. Overall Chi Square test of significance is .005, better detection as compared to Peak enhancement phase.
A
| DEMR | |||||
|---|---|---|---|---|---|
| Adenoma | Atypical | Carcinoma | Total | ||
| CEUS Adenoma | Number | 15 | 0 | 0 | 15 |
| % | 75.00% | 0.00% | 0.00% | 75.00% | |
| CEUS Atypical | Number | 0 | 1 | 0 | 1 |
| % | 0.00% | 5.00% | 0.00% | 5.00% | |
| CEUS Carcinoma | Number | 0 | 1 | 3 | 4 |
| % | 0.00% | 5.00% | 15.00% | 20.00% | |
| Total | Number | 15 | 2 | 3 | 20 |
| % | 75.00% | 10.00% | 15.00% | 100% | |
B
| Category | Value | df | Asymp-Sig (12- sided) P-Value |
|---|---|---|---|
| Pearson Chi-Square | 27.5 | 4 | 0.005 |
| Likelihood ratio | 24.725 | 4 | 0 |
| No. of valid cases | 20 | - | |
Table 2: (A, B) Showing cross tab value of CEUS and DEMR with Chi-Square test (A) Time to peak enhancement phase (B) Chi-Square test of significance for time to peak enhancement.
Table 3 is showing in phase by cross tabulation and Chi square test comparison with gold standard biopsy correlation. Average time taken for contrast to reach the nodule after injection. There is strong correlation in detecting adenoma by CEUS and Dynamic MRI, around 80.0% for In-phase enhancement. There is weak correlation in detecting atypical lesions and carcinoma by CEUS and DEMR around 10.0% and 10.0%. Overall Chi Square test significance is .005.
A
| DEMR | |||||
|---|---|---|---|---|---|
| Adenoma | Atypical | Carcinoma | Total | ||
| CEUS Adenoma | Number | 16 | 0 | 0 | 16 |
| % | 80.00% | 0.00% | 0.00% | 80.00% | |
| CEUS Atypical | Number | 0 | 2 | 0 | 2 |
| % | 0.00% | 10.00% | 0.00% | 10.00% | |
| CEUS Carcinoma | Number | 0 | 2 | 2 | 4 |
| % | 0.00% | 0.00% | 10.00% | 10.00% | |
| Total | Number | 16 | 2 | 3 | 20 |
| % | 80.00% | 10.00% | 10.00% | 100% | |
B
| Category | Value | df | Asymp-Sig (12- sided) P-Value |
|---|---|---|---|
| Pearson Chi-Square | 40 | 4 | 0.005 |
| Likelihood ratio | 25.561 | 4 | 0 |
| No. of valid cases | 20 | - | |
Table 3: (A, B) Showing cross tab value of CEUS and DEMR with Chi-Square test (A) Wash in enhancement phase (B) Chi-Square test of significance for wash in enhancement.
Table 4 is showing out phase by cross tabulation and chi-square test comparison with gold standard biopsy correlation. Average taken for contrast to wash out from nodule. There is strong correlation in detecting adenoma by CEUS and Dynamic MRI, around 88.9% for outphase enhancement. Out phase enhancement in diagnosing atypical lesion by CEUS and DEMR-0%, not reliable detection rate. Overall Chi Square test significance is higher than other phase .007, due to higher Sensitivity in diagnosing adenoma.
A
| DEMR | |||||
|---|---|---|---|---|---|
| Adenoma | Atypical | Carcinoma | Total | ||
| CEUS Adenoma | Number | 16 | 0 | 0 | 16 |
| % | 88.90% | 0.00% | 0.00% | 88.90% | |
| CEUS Atypical | Number | 0 | 0 | 0 | 0 |
| % | 0.00% | 0.00% | 0.00% | 0.00% | |
| CEUS Carcinoma | Number | 0 | 0 | 2 | 2 |
| % | 0.00% | 0.00% | 11.10% | 11.10% | |
| Total | Number | 16 | 0 | 2 | 18 |
| % | 88.90% | 0.00% | 11.10% | 100% | |
B
| Category | Value | df | Asymp-Sig (12- sided) P-Value |
|---|---|---|---|
| Pearson Chi-Square | 18 | 1 | 0.007 |
| Likelihood ratio | 12.558 | 4 | 0 |
| No. of valid cases | 18 | - | |
Table 4: (A, B) Showing cross tab value of CEUS and DEMR with Chi-Square test (A) Wash out enhancement phase (B) Chi-Square test of significance for wash out enhancement.
By statistical percentage analysis, CEUS versus HPE correlation comes to Sensitivity 66.67% and Specificity 94.44%. DEMR versus HPE correlation of Sensitivity ratio is 66.67% and Specificity is 100%. DEMR scores over CEUS in minimizing false positive ratio. By combining both modalities advanced protocol, Specificity goes to 100% in ruling out false positive diagnosis.
Discussion
In our study, four phase of contrast enhancement correlated in CEUS and DEMR, out of all subtypes pickup rate of adenoma remains high, followed by carcinoma detection and last atypical lesion detection. Pitfalls encountered in detecting atypical cyst due to variable enhancement, especially in out phase category where no sign of enhancement and detection obtained. Among the modality of choices in working up parathyroid incidentaloma, Ultrasound good accuracy but operator dependent less reliable for ectopic lesions and superior pole lesions ultrasound (59-89%) sensitivity, radiation (0). Wherein sestamibi (54%-89%)-sensitivity (63%), radiation 7.8 msv (3,4), 4D CT 65%-88% Sensitivity, radiation 10.4 msv, less favored in young patients [4-6].
Diffusion weighted signal is produced from the movement of water in the intra, and extra cellular spaces and also from intravascular spaces. Diffusion combined with DEMR do have significant role with better sensitivity in diagnosing solitary parathyroid nodules [7,5].
Four-dimensional CT has advantages common to both US and scintigraphy. It provides excellent anatomic detail for preoperative localization in ectopic locations, while the multiple phases show uptake characteristics that help to differentiate parathyroid lesions from lymph nodes and thyroid nodules [10,11].
Roy et al. assessed the sensitivity of ultrasound and scintigraphy in the detection of ectopically located parathyroid adenomas The main advantages of scintigraphy include higher sensitivity compared to ultrasound in detecting ectopic lesions in the thymus, retroesophageal, thyroid, mediastinum, undescended (situated at least 1 cm above the upper thyroid pole) as well as in the vicinity of carotid arteries. The sensitivity of ultrasound and scintigraphy for the detection of this type of lesions was 59% and 89%, respectively SPECT was found to further increase the sensitivity of parathyroid imaging from 87% (as in planar scintigraphy) up to 95% [20]. The introduction of hybrid SPECT/ CT, an instrument that physically couples a SPECT camera with a CT in a single integrated unit offers the potential advantage of better anatomically defining the location of scintigraphic findings that are identified on SPECT images. Multiparametric MR dynamic perfusion can be used to exploit the hypervascular nature of PTAs to distinguish them from subjacent thyroid tissue or lymph nodes with high accuracy [18,19]. MR perfusion analysis, a combination of TTP (threshold of 30 seconds), wash-in (threshold of 5.86), and washout (threshold of 0.67) improved the diagnostic power of better sensitivity/specificity of 91%/95% [21-23].
Primary hyperparathyroidism can be cured with resection of the overactive gland. Single parathyroid adenoma is now most commonly resected through a smaller unilateral incision on one side of the neck in one quadrant. Preoperative localization of hyperfunctioning parathyroid lesions-role of radiologist and endocrine surgeon is challenging task [24-26].
In our study, combining peak enhancement (total time of contrast from entry to exit with different mean for different pathology, adenoma (55 sec), carcinoma (45 sec) and atypical mixed values and Time to peak (average time 40 seconds for adenoma wherein carcinoma relatively less) yielded good confidence interval. Wash In and Wash Out ratio combined do not yield significant results in CEUS and DEMR [27-30].
To best of our knowledge, our study first ever combined CEUS and DEMR characteristics in establishing better specificity of diagnosing solitary parathyroid incidentaloma.
Bilateral neck exploration has been a widely used treatment method for adenomas in twentieth century. In the last decade, however, it has been increasingly replaced by a minimally invasive surgical treatment. In future prospects with innovative noninvasive and non-radiating image planning, localization rate of even small nodule and pathological diagnosis have significant value on minimal invasive procedure.
Limitation in our study was using different types of contrast and minimal injection related morbidity. No significant adverse reactions noted in none of our patients. Contrast mediated reactions are very rare possibilities [31-38].
Conclusion
Dynamic 4D contrast-enhanced MRI and Contrast enhanced ultrasonography combined with high temporal and spatial resolutions can now be obtained for pre-operative identification of PTI (Parathyroid Incidentaloma) and differentiating from other lesions. The radiologist’s ability to detect to differentiate it from mimics and false positive cases can also be increased with appreciation of the typical contrast enhancement characteristics by combining CEUS and DEMR.
Conflict of Interest
The authors declares no conflict of interest.
References
- Heath H, Hodgson SF, Kennedy MA (1980) Primary hyperparathyroidism: Incidence, morbidity, and potential economic impact in a community. New Eng J Med 302: 189-193.
- Chen H, Mack E, Starling JR (2005) A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg 242: 375.
- Doppman JL (1976) Parathyroid localization: arteriography and venous sampling. Rad Clin North America 14: 163-188.
- Miller DL, Doppman JL (1987) Parathyroid angiography. Ann Int Med 107: 942-943.
- Mortenson MM, Evans DB, Lee JE, Hunter GJ, Shellingerhout D, et al. (2008) Parathyroid exploration in the reoperative neck: Improved preoperative localization with 4D-computed tomography. J Am Coll Surg 206: 888-895.
- Kelly HR, Hamberg LM, Hunter GJ (2014) 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: Accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naive and re-exploration patients. Am J Neurorad 35: 176-181.
- Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, et al. (2012) Parathyroid four-dimensional computed tomography: Evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg 36: 1335-1339.
- Madorin CA, Owen R, Coakley B, Lowe H, Nam KH, et al. (2013) Comparison of radiation exposure and cost between dynamic computed tomography and sestamibi scintigraphy for preoperative localization of parathyroid lesions. JAMA Surg 148: 500-503.
- Gotway MB, Reddy GP, Webb WR, Morita ET, Clark OH, et al. (2001) Comparison between MR imaging and 99mTc MIBI scintigraphy in the evaluation of recurrent or persistent hyperparathyroidism. Radiol 218: 783-790.
- Lee VS, Spritzer CE, Coleman RE, Wilkinson H, Coogan AC, et al. (1996) The complementary roles of fast spin-echo MR imaging and double-phase 99m TC-sestamibi scintigraphy for localization of hyperfunctioning parathyroid glands. Am J Roentgenol 167: 1555-1562.
- Grayev AM, Gentry LR, Hartman MJ, Chen H, Perlman SB, et al. (2012) Presurgical localization of parathyroid adenomas with magnetic resonance imaging at 3.0 T: An adjunct method to supplement traditional imaging. Ann Surg Oncol 19: 981-989.
- Song T, Laine AF, Chen Q, Rusinek H, Bokacheva L, et al. (2009) Optimal kâ€space sampling for dynamic contrastâ€enhanced MRI with an application to MR renography. Mag Reson Med 61: 1242-1248.
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, et al. (2005) Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multiâ€slice imaging. Mag Reson Med 53: 684-691.
- Dixon WT (1984) Simple proton spectroscopic imaging. Radiol 153: 189-194.
- Barger AV, DeLone DR, Bernstein MA, Welker KM (2006) Fat signal suppression in head and neck imaging using fast spin-echo-IDEAL technique. Am J Neurorad 27: 1292-1294.
- Le Y, Kroeker R, Kipfer HD, Lin C (2012) Development and evaluation of TWIST Dixon for dynamic contrastâ€enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Mag Reson Imag 36: 483-491.
- Tofts PS (1997) Modeling tracer kinetics in dynamic Gdâ€DTPA MR imaging. J Mag Reson Imag 7: 91-101.
- Haker S, Wells WM, Warfield SK, Talos IF, Bhagwat JG, et al. (2005) Combining classifiers using their receiver operating characteristics and maximum likelihood estimation. In: International Conference on Medical Image Computing and Computer-Assisted Intervention 506-514.
- Linda DD, Ng B, Rebello R, Harish S, Ioannidis G, et al. (2012) The utility of multidetector computed tomography for detection of parathyroid disease in the setting of primary hyperparathyroidism. Can Ass Radiol J 63: 100-108.
- Gafton AR, Glastonbury CM, Eastwood JD, Hoang JK (2012) Parathyroid lesions: Characterization with dual-phase arterial and venous enhanced CT of the neck. Am J Neuroradiol 33: 949-952.
- Noureldine SI, Aygun N, Walden MJ, Hassoon A, Gujar SK, et al. (2014) Multiphase computed tomography for localization of parathyroid disease in patients with primary hyperparathyroidism: How many phases do we really need? Surgery 156: 1300-1307.
- Kutler DI, Moquete R, Kazam E, Kuhel WI (2011) Parathyroid localization with modified 4Dâ€computed tomography and ultrasonography for patients with primary hyperparathyroidism. Laryngoscope 121: 1219-1224.
- Harari A, Zarnegar R, Lee J, Kazam E, Inabnet WB, et al. (2008) Computed tomography can guide focused exploration in select patients with primary hyperparathyroidism and negative sestamibi scanning. Surgery 144: 970-977.
- Chazen JL, Gupta A, Dunning A, Phillips CD (2012) Diagnostic accuracy of 4D-CT for parathyroid adenomas and hyperplasia. Am J Neurorad 33: 429-433.
- Starker LF, Mahajan A, Björklund P, Sze G, Udelsman R, et al. (2011) 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol 18: 1723-1728.
- Beland MD, Mayo-Smith WW, Grand DJ, Machan JT, Monchik JM (2011) Dynamic MDCT for localization of occult parathyroid adenomas in 26 patients with primary hyperparathyroidism. Am J Roentgenol 196: 61-65.
- Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, et al. (2006) Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 140: 932-941.
- Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, et al. (2010) American association of clinical endocrinologists, guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive summary of recommendations. J Endocrinol Invest 33: 51-56.
- Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, et al. (2005) Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiol 237: 794-800.
- Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, et al. (2006) Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: Initial results. Eur Radiol 16: 2234-2241.
- Nemec U, Nemec SF, Novotny C, Weber M, Czerny C, et al. (2012) Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: Assessment of diagnostic accuracy. Eur Radiol 22: 1357-1365.
- Hornung M, Jung EM, Georgieva M, Schlitt HJ, Stroszczynski C, et al. (2012) Detection of microvascularization of thyroid carcinomas using linear high resolution contrast-enhanced ultrasonography (CEUS). Clin Hemorheol Microcircul 52: 197-203.
- Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, et al. (2010) Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid 20: 51-57.
- Friedrich-Rust M, Sperber A, Holzer K, Diener J, Grünwald F, et al. (2010) Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diab 118: 602-609.
- Xu HX (2009) Contrast-enhanced ultrasound: The evolving applications. World J Radiol 1: 15‑24.
- Giusti M, Orlandi D, Melle G, Massa B, Silvestri E, et al. (2013) Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Uni SCI B 14: 195-206.
- Zheng XJ, Zhang YK, Zhao CY, Liang JR, LE H, et al. (2010) Enhancement pattern of thyroid carcinoma with contrast-enhanced ultrasound. Zhonghua yi xue za zhi 90: 42-45.
Citation: Ameen MD, Kumaran S, Mani K (2018) A Combined Approach of CEUS and DEMR in the Diagnosis of Parathyroid Hyperparathyroidism. OMICS J Radiol 7: 297. DOI: 10.4172/2167-7964.1000297
Copyright: © 2018 Ameen MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 5165
- [From(publication date): 0-2018 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 4353
- PDF downloads: 812