A Clinicopathological Study of cd34 Antigen Expression in Benign and Malignant Breast Lesions
Received: 07-Aug-2017 / Accepted Date: 12-Aug-2017 / Published Date: 16-Aug-2017 DOI: 10.4172/2161-0681.1000321
Abstract
Objectives: To study stromal CD34 expression in benign and malignant breast lesions and evaluate whether loss of CD34 is specific for invasive disease.
Material and methods: 317 cases of benign and malignant breast lesions were studied over a period of 2 years (July 2012 to June 2014). All the cases underwent routine processing followed by H and E staining. 178 cases underwent CD34 immunohistochemical staining and semi quantitative assessment. Further, 74 cases of infiltrating ductal carcinoma were evaluated to determine relation of stromal CD34 expression with histological grade and lymph node metastasis.
Results: Normal breast showed retained CD34 expression in 90.9% cases. Among benign lesions, 83% cases showed retained stromal CD34 expression (except cases of apocrine metaplasia, intraductal papilloma and fibroadenoma with focal atypia which showed reduced expression in 46.67%, 100% and 33.3% cases respectively). Both DCIS and LCIS showed reduced expression. Malignant tumors showed grade 0 staining in 97.9% cases, irrespective of the histological type. Statistically significant differences in stromal CD34 expression were observed between:
Malignant phyllode`s tumor compared to its benign counterpart
Papilloma and papillary carcinoma
Benign and malignant lesions overall
No significant correlation was found between CD34 expression and histological grade or lymph node metastasis. The metastatic foci in node positive patients showed a similar pattern of CD34 expression in the lymph nodes as those seen in the breast.
Conclusion: Owing to the significant differences in CD34 expression between benign and malignant breast lesions, it can potentially be used to differentiate between the two and can serve as an important diagnostic marker. Further studies can also be undertaken to establish its role as a therapeutic target in cases of breast cancer.
Keywords: Breast; Cancer; Stroma; Myofibroblasts; CD34
316116Introduction
There is increasing evidence to indicate that the tumor microenvironment exerts a major modulatory effect on epithelial tumors and the stroma makes an important contribution to the process of tumor progression [1]. In breast, it influences growth, differentiation, invasive behavior and polarity of normal mammary epithelial cells, as well as those in breast carcinomas.
In several systems, the stroma has been shown to have a profound effect on normal and tumor cell behavior [2-4] and it has been suggested that stroma may play a dominant role in regulating breast epithelial cell function [5]. The stroma around invasive breast tumours is known to differ from normal breast, with alterations in stromal protein synthesis [6] and expression of MMP [7,8]. Normal mammary stroma has huge numbers of CD34 fibrocytes [9]. However, in breast carcinoma: CD34 fibrocytes undergo alterations with adoption of a plump myofibroblast-like appearance and loss of CD34 expression, accompanied by the acquisition of alpha-smooth muscle actin (SMA) expression [10]. This is known as myofibroblastic differentiation.
“It has been suggested that there is an inverse relation between CD34 expression and myofibroblastic differentiation” [11].
CD34 is a transmembrane glycoprotein that is thought to be involved in the modulation of cell adhesion and signal transduction [12] and is expressed by mesenchymal cells at several sites, including the normal mammary stroma [13]. Loss of CD34 by mesenchymal cells has been described in several other situations where there is malignant transformation of the mesenchymal population. Malignant phyllodes tumors of the breast exhibit lower levels of CD34 expression than benign phyllodes or fibroadenoma [14-16] and CD34 is lost in sarcomas arising within CD34 positive dermatofibrosarcoma protruberans [17]. Loss of fibroblast CD34 has also been described in non-neoplastic fibroblasts around epithelial tumors, such as basal cell carcinoma [18] and colorectal carcinoma [19].
The above mentioned studies formed the basis of the present study evaluating CD34 expression in stroma of benign and malignant breast lesions. This study aimed to determine whether loss of CD34 is specific for invasive disease in the breast also (as has already been proved in other organs). An indication to this conclusion has been provided by some earlier researchers [11-22]. Bucala R et al. also postulated that since CD34+ fibrocytes are capable of collagen I and collagen III synthesis, it seems to be likely that this cell type may be involved in the process of stromal remodeling in breast cancer [23]. Conclusive proof of loss of CD34 in breast carcinoma would not only lead to the identification of a new marker to diagnose cases of breast carcinoma, but could also lead to the generation of a putative therapeutic target in the years to come. The present study is, therefore, aimed to study CD34 expression in the stroma of benign and malignant breast lesions and to discuss the role of CD34 in differentiation of benign and malignant diseases of the breast.
Material and Methods
The present study was carried out on 317 patients of benign and malignant breast lesions, attending the OPD/IPD of Department of Surgery and on the histopathological specimens received in the Department of Pathology, J. N. Medical College, AMU, Aligarh. The study was conducted over a period of 2 years (from July 2012 to June 2014). A detailed clinical history and examination, along with routine investigations were carried out in each case.
Post-surgical specimens included mastectomies and a few biopsies. Gross examination of all the specimens was performed and sections were taken from the representative areas. They were processed by an automatic tissue processor (Histokinette). Blocks were prepared in paraffin wax with the help of Paraffin Embedding Station. Sections were cut at 4-5 μm thickness with the help of rotary microtome. All 317 cases were stained by Haematoxylin and Eosin and examined microscopically. Of these, 178 representative cases were selected for CD34 immunostaining.
Immuno-histochemistry for CD34 was performed on paraffin embedded tissue sections using the kit, Thermo Scientific CD34 (Clone QBEnd/10). The antibody provided is prediluted and ready to use. Tissue was first microwaved in citrate buffer (at pH- 6.0, 95ºC, 10 min) for antigen retrieval. This was followed by peroxide block, protein block and incubation in primary antibody for 20 min at room temperature. Further incubation with HRP polymer and DAB was done. Finally, counterstaining with haematoxylin was done and dried, mounted slides were examined microscopically.
Each section was examined and the number of duct/ lobular units was identified. The grading of CD34 expression was determined for each duct/ lobular unit separately. The sections stained for CD34 were evaluated at high power (400X microscopic field; objective 40X, eyepiece 10X). Grading was done from 0 to 3+, where
0: upto 5% stromal cells immunoreactive
1+: >5 and upto 25% stromal cells immunoreactive
2+: >25 and upto 50% stromal cells immunoreactive
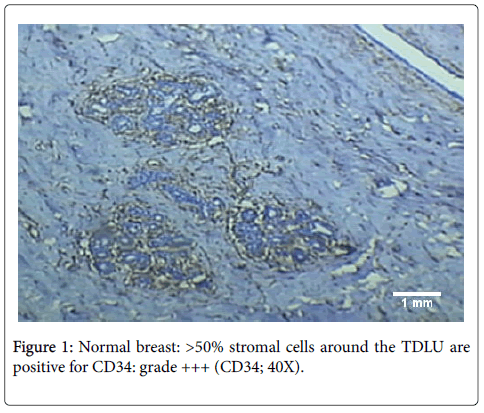
3+: >50% stromal cells immunoreactive
Assuming that a high power microscopic field harbored 100 stromal cells. The staining of endothelial cells in blood vessels was taken as internal control.
Grade 0- was interpreted as complete loss of CD34
Grade 1+ -was interpreted as reduced expression,
While grade 2+ and 3+ were interpreted as retained expression of CD34.
The tests of significance used were chi square test, test of proportions and student`s t test.
Observations
The 317 cases of breast lesions were divided into 2 broad categories-non neoplastic (41 cases, 13%) and neoplastic (276 cases, 87%). Neoplastic lesions were further subdivided into benign neoplasms (110 cases, 39.8%) and malignant neoplasms (166 cases, 60.1%). Most of the cases in non-neoplastic category were of fibrocystic disease (25 cases, 60.9%) followed by duct ectasia (17%), chronic mastitis (17%) and acute mastitis (4.9%). Fibroadenoma formed the largest group in benign neoplasms (93 cases, 84.5%). This was followed in frequency by gynaecomastia (10.9%), benign phyllode`s tumor (2.7%) and papillomas (1.8%). Various subtypes of fibroadenomas included were juvenile fibroadenoma (10 cases), fibroadenomatoid hyperplasia (9 cases), 2 cases of fibroadenoma with atypical changes/ADH, 4 cases of fibroadenoma with fibrocystic changes and 3 cases of complex fibroadenoma.
Among the malignant neoplasms, IDC was the most common (147 cases, 88.5%). Medullary carcinoma (6 cases), and a single case of tubular carcinoma and IDC with mucinous change each, were included in this category. IDC was followed by metaplastic carcinoma (3.6%), ILC (3%), Paget`s disease (1.8%), malignant phyllode`s tumor (1.2%), poorly differentiated carcinoma (1.2%) and intracystic papillary carcinoma (0.6%). 2 cases of infiltrating ductal carcinoma mixed with infiltrating lobular carcinoma were also seen. Besides the above, ductal and lobular hyperplasias, DCIS, LCIS, apocrine metaplasia and atypical hyperplasia were also seen associated with these lesions.
CD34 expression in normal breast was studied in the normal areas of sections of breasts with lesions. For example, all the cases with a diagnosis of acute and chronic mastitis showed normal TDLUs and were included in this category. Normal TDLUs were also seen in various other sections.
Most of the patients were females in the age group of 31 to 40 years who presented with a breast lump. 14.6% and 27% of the malignant cases showed skin involvement and base involvement respectively while about 85% showed involvement of 1 or more margins. Involvement of nipple areola and lymphovascular and/or perineural involvement was seen in 20% and 27% cases respectively.
61 of the total 157 malignant tumors (38.9%) showed lymph node metastasis to ipsilateral axillary lymph nodes. On histological grading of the 141 infiltrating ductal carcinomas (excluding 6 cases received as biopsies), 73 cases (51.7%) were grade 2, 43 (30.5%) were grade 3 and 25 (17.7%) were grade 1. 38.7% of all malignant tumors were stage II on TNM staging, 35.4% were stage I, 14.8% were stage III and 10.9% were stage IV.
A total of 178 cases were selected and subjected to immunohistochemical staining for CD34. The number of cases selected in each category is shown in Table 1. Each individual case was studied and the number of duct/lobular units in each case was calculated. CD34 staining was assessed for each duct/lobular unit separately as the changes in CD34 were found to be highly localized with separate areas in the same section showing completely different staining patterns. Table 2 shows the cumulative number of duct/ lobular units with a specific diagnosis and their pattern of CD34 staining while Table 3 shows comparison of CD34 expression between benign and malignant lesions overall.
| Nature | Lesion | No. of cases |
|---|---|---|
| Non neoplastic 27 cases (15.2%) | Duct ectasia | 5 (18.5) |
| Acute mastitis | 2 (7.4) | |
| Chronic mastitis | 12 (44.4) | |
| Fibrocystic disease | 8 (29.6) | |
| Neoplastic 151 cases (84.8%) |
BENIGN: 58 cases (38.4%) | |
| Fibroadenoma | 47 (81) | |
| Gynaecomastia | 6 (10.3) | |
| Benign phyllode`s tumor | 3 (5.2) | |
| Papilloma | 2 (3.4) | |
| Malignant: 93 cases (61.5%) | ||
| IDC | 74 (79.6) | |
| ILC | 5 (5.3) | |
| Malignant phyllode`s tumor | 2 (2.1) | |
| Intracystic papillary carcinoma | 1 (1.1) | |
| Metaplastic carcinoma | 6 (6.5) | |
| Poorly differentiated carcinoma | 2 (2.1) | |
| Paget`s disease | 3 (3.2) | |
| Total | 178 | |
| Figures in parentheses indicate percentages. | ||
Table 1: Cases selected for immunostaining.
| Histology | Total no. duct/lobular units | CD34 | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| Normal | 276 | 0 | 0 | 25 | 251 |
| Non neoplastic | |||||
| Duct ectasia | 79 | 0 | 0 | 15 | 64 |
| Apocrine metaplasia | 90 | 0 | 42 | 38 | 10 |
| Adenosis | 147 | 0 | 0 | 17 | 130 |
| fibrocystic disease | 488 | 0 | 0 | 7 | 481 |
| Fibroadenoma | |||||
| Fibroadenoma | 1596 | 0 | 0 | 167 | 1429 |
| Juvenile fibroadenoma | 180 | 0 | 0 | 13 | 167 |
| Complex fibroadenoma | 30 | 0 | 10 | 20 | 0 |
| Fibroadenoma with focal atypia | 51 | 16 | 18 | 14 | 3 |
| Gynaecomastia | 85 | 0 | 0 | 14 | 71 |
| Phyllode`s tumor | |||||
| Benign phyllode`s tumor | 69 | 0 | 12 | 31 | 26 |
| Borderline phyll. tm | 50 | 0 | 20 | 30 | 0 |
| Malignant phyllode`s tm | 9 | 4 | 1 | 4 | 0 |
| Papillary lesions | |||||
| Intraductal papilloma | 2 | 0 | 0 | 2 | 0 |
| Papillary carcinoma | 13 | 13 | 0 | 0 | 0 |
| Epithelial hyperplasias | |||||
| UDH | 103 | 0 | 0 | 21 | 82 |
| ADH | 25 | 0 | 1 | 7 | 17 |
| lobular hyperplasia | 27 | 0 | 0 | 3 | 24 |
| In situ carcinoma | |||||
| DCIS (low grade) | 127 | 0 | 10 | 49 | 68 |
| DCIS (high grade) | 208 | 69 | 111 | 28 | 0 |
| LCIS | 26 | 3 | 12 | 10 | 1 |
| Malignant tumors | |||||
| IDC | 3479 | 3409 | 70 | 0 | 0 |
| ILC | 263 | 253 | 10 | 0 | 0 |
| Medullary carcinoma | 158 | 158 | 0 | 0 | 0 |
| Tubular carcinoma | 43 | 43 | 0 | 0 | 0 |
| Metaplastic carcinoma | 218 | 217 | 1 | 0 | 0 |
| Poorly differentiated carcinoma | 45 | 45 | 0 | 0 | 0 |
Table 2: Summary of the pattern of expression of CD34 in fibroblasts in relation to histopathological features.
| Histology | TDLU | CD34 staining | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| Benign | |||||
| Duct ectasia | 79 | 0 | 0 | 15 | 64 |
| Apocrine metaplasia | 90 | 0 | 42 | 38 | 10 |
| Adenosis | 147 | 0 | 0 | 17 | 130 |
| fibrocystic disease | 488 | 0 | 0 | 7 | 481 |
| Fibroadenoma | 1596 | 0 | 0 | 167 | 1429 |
| Juvenile fibroadenoma | 180 | 0 | 0 | 13 | 167 |
| Complex fibroadenoma | 30 | 0 | 10 | 20 | 0 |
| Fibroadenoma with focal atypia | 51 | 16 | 18 | 14 | 3 |
| Gynaecomastia | 85 | 0 | 0 | 14 | 71 |
| Benign phyllode`s tumor | 69 | 0 | 12 | 31 | 26 |
| Borderline phyllode`s tm | 50 | 0 | 20 | 30 | 0 |
| UDH | 103 | 0 | 0 | 21 | 82 |
| ADH | 25 | 0 | 1 | 7 | 17 |
| lobular hyperplasia | 27 | 0 | 0 | 3 | 24 |
| Total | 3020 | 16 (0.5) | 103 (3.4) | 397 (13.1) | 2504 (83) |
| Malignant | |||||
| Malignant phyllode`s tm | 9 | 4 | 1 | 4 | 0 |
| Intracystic papillary carcinoma | 13 | 13 | 0 | 0 | 0 |
| IDC | 3479 | 3409 | 70 | 0 | 0 |
| ILC | 263 | 253 | 10 | 0 | 0 |
| Medullary carcinoma | 158 | 158 | 0 | 0 | 0 |
| Tubular carcinoma | 43 | 43 | 0 | 0 | 0 |
| Metaplastic carcinoma | 218 | 217 | 1 | 0 | 0 |
| Poorly differentiated carcinoma | 45 | 45 | 0 | 0 | 0 |
| Total | 4228 | 4142 (97.9) | 82 (1.9) | 4 (0.09) | 0 (0) |
| Figures in parentheses indicate percentage. | |||||
Table 3: Comparison of CD34 expression in benign and malignant breast lesions.
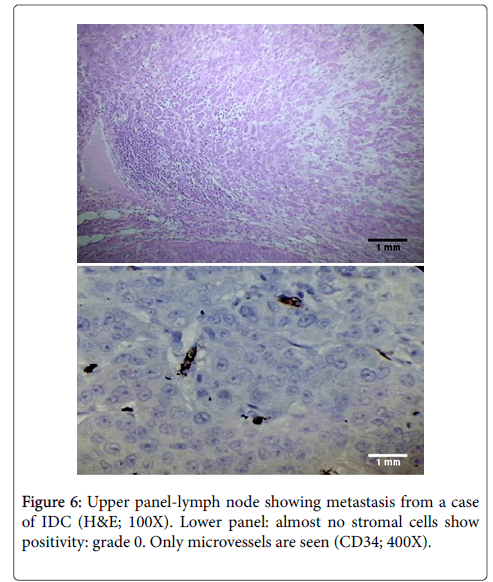
CD34 expression was also studied in 44 cases of lymph nodes which showed metastasis from breast carcinoma. Most of these metastatic foci were from infiltrating ductal carcinoma. Others were from 1 case with mixed infiltrating ductal and lobular carcinoma, 3 cases of medullary carcinoma, 3 cases of infiltrating lobular carcinoma, and 2 cases of metaplastic carcinoma. The metastatic foci showed a similar pattern of CD34 expression in the lymph nodes as those seen in the breast. There was loss of CD34 in 39 of 44 cases (88.6%) while rest of the cases (5 of 44) showed CD34 staining grade of 1+.
Relation of stromal CD34 staining with presence or absence of lymph node metastasis and histological grade was also studied to establish the role of CD34 as a prognostic marker in breast cancer. We aimed to determine whether decreased expression of CD34 in breast cancer is related to poor tumor characteristics, i.e., high histological grade and presence of lymph node metastasis. This study was done in 74 cases of infiltrating ductal carcinoma (with or without DCIS) only. Cases of ILC, medullary carcinoma, tubular carcinoma, metaplastic carcinoma and poorly differentiated carcinoma were not included.
Table 4 summarizes our findings. As shown in the table, there seemed to be no relation between loss of CD34 and histological grade as both low grade and high grade lesions showed similar frequency of loss of CD34. Similarly, there was little difference in CD34 expression between node positive and node negative patients.
| Parameter | Percentage of cases with loss of CD34 | Total number of cases |
|---|---|---|
| Lymph node metastasis + | 98% | 35 |
| Lymph node metastasis - | 97% | 39 |
| P value=0.76, not significant | ||
| Histological Grade 1 | 97.5% | 14 |
| Histological Grade 3 | 98.01% | 20 |
| P value=0.84, not significant | ||
Table 4: Relation of CD34 with lymph node metastasis and histological grade.
Discussion
CD34, a 110-kDa transmembrane cell-surface glycoprotein, has been identified as a marker of human hematopoietic cells [12]. CD34- positive stromal cells are also distributed in various normal organs including salivary gland, thyroid gland, tonsil, stomach, colon, uterus, Fallopian tube and testis [13-29]. Several studies have been conducted to investigate the distribution of CD34-positive stromal cells in neoplasms of various organs including salivary gland, stomach, colorectal tissue, breast, pancreas and uterine cervix [11-31].
CD34-positive stromal cells are present in the normal breast [11]. In our study diffuse CD34 expression was seen in the interlobular as well as intralobular stroma of the normal breast (Figure 1). However, the origin of these stromal cells remains controversial with some researchers advocating that CD34+ fibroblasts are present in stroma since birth [11], while others are of the view that they are derived from blood borne fibrocytes [21].
Some investigators have reported that CD34 positive stromal cells disappear in the stroma of invasive ductal carcinoma (IDC) of the breast [11-32]. These observations formed the basis of our study. The changes in CD34 expression in breast lesions have been found to be much localized in earlier studies with loss around areas of IDC, but retained expression in adjacent normal breast glands. This finding has been proved in our study also. This is an important finding as it implicates epithelial mesenchymal interactions in control of expression of CD34 [20].
Benign lesions
Most of the benign lesions in our study showed a similar pattern of CD34 expression with dense, diffuse staining for CD34 in the stroma (grade 2+ to 3+). Similar findings have been demonstrated by different researchers earlier [11-22]. However, few of the benign lesions in our study showed a different staining pattern than that described by previous researchers, as discussed below.
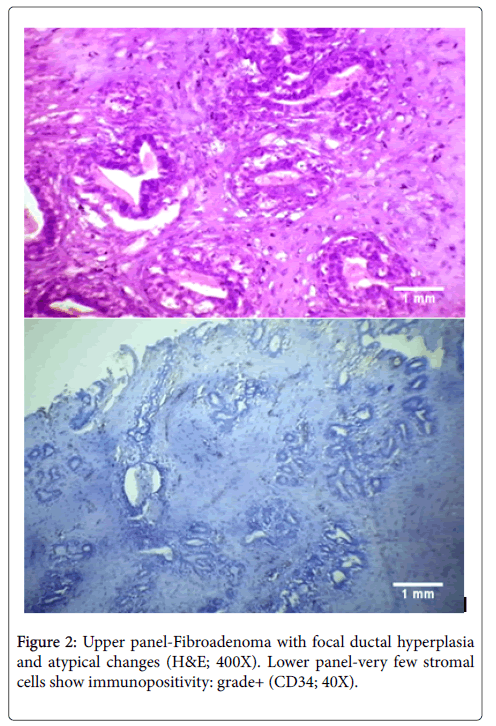
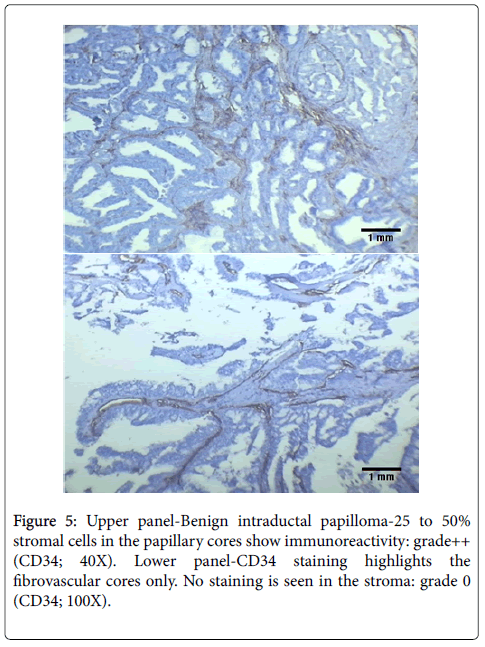
The cases of atypical ductal hyperplasia included in our study showed grade 2+ to 3+ expression in 96% cases (24/25 cases) with reduced expression in one case only. In comparison Chauhan et al. demonstrated in their study that 50% of cases of ADH showed diffuse staining for CD34 and 50% had partial loss around a duct [20]. However, in another study performed in 2005, Cimpean et al. found the staining pattern of ADH to be similar to that found in our study, with 100% cases showing retained CD34 expression [22]. We found that the areas with apocrine metaplasia in our study showed decreased staining as compared to other benign lesions with 53.33% showing grade 2+ to 3+ staining and 46.67% showing reduced CD34 expression. This was similar to the findings of Cimpean et al. who also had a single case of apocrine metaplasia in their study which showed loss of CD34 expression [22]. We also had 2 cases of fibroadenoma with focal atypical changes in our study. Both of these cases showed localized reduction of CD34 expression around the areas with atypia (Figure 2). This was similar to the findings of Cimpean et al. who also demonstrated loss of CD34 expression in a case of fibroadenoma with malignant change [22]. The status of CD34 expression in intraductal papilloma has not been studied much. Kuroda et al. showed negative to focal staining in cases of papilloma, in contrast to other benign lesions [33]. We also found in the present study that the cases of intraductal papilloma showed focal staining which was lower than that in other benign lesions with a grade of 1+ to 2+. Cimpean et al. however, demonstrated no change in papilloma from other benign lesions; with retained expression in all the cases [22].
The reduced expression in case of fibroadenoma with malignant change can be explained due to the localized changes in stroma owing to the increased malignant potential in this case. However, the reasons for reduced CD34 expression in the other two cases remain unclear and it remains to be seen whether this pattern suggests a premalignant potential in cases of apocrine metaplasia and intraductal papilloma. It is important to mention here that though presence of a single isolated papilloma is not associated with increased chances of malignancy, the presence of multiple papillomas and those with atypical changes have been proven to be associated with an increased frequency of development of breast carcinoma [34]. Hence, the findings in our study could be a manifestation of the premalignant differentiation in these cases.
Carcinoma in situ
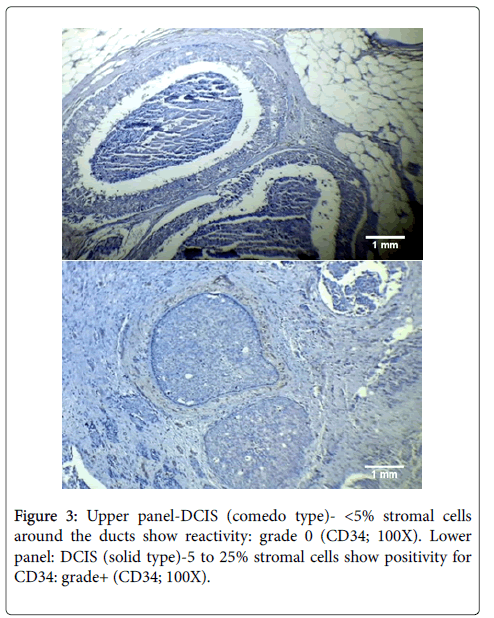
The pattern of CD34 expression in cases of DCIS remains unclear with different researchers demonstrating different findings in the past. A comparison of our findings in DCIS with those of different researchers earlier is given below.
| BARTH, 2002 | CIMPEAN, 2005 | CHAUHAN, 2003 | CATTEAU, 2013 | PRESENT STUDY | |
|---|---|---|---|---|---|
| CD34 expression | CD34 expression | CD34 expression | CD34 expression | CD34 expression | |
| DCIS | 0% | 0% | Low: 60% | Low: 100% | Low: 92% |
| Intermediate:62% | Intermediate:95% | High: 13% | |||
| High: 22% | High: 94% |
In our study, the difference in loss of CD34 expression between low and high grade DCIS was found to be highly significant statistically (p<0.001). Thus, our findings demonstrated a decrease in the pattern of CD34 expression with increasing grade in cases of DCIS (see Figure 3). This change in the pattern of CD34 expression in higher grades of DCIS can very well be explained by the increased malignant potential of high grade DCIS. This is verified by the fact that there is a substantial difference in the frequency of evolution to invasive carcinomas depending on the type of DCIS: high chances of developing invasive cancer in high grade lesions and low chances in all others [35].
Considering LCIS: a proportion of the cases of lobular carcinoma in situ included in our study (11.5%) showed loss of expression of CD34. This finding was different from those of Chauhan et al. and Cimpean et al. who found retained expression in 100% cases [20,22]. The nature of LCIS with regards to the probability of its subsequent evolution to invasive carcinoma remains controversial. However, there is a consensus that approximately 20 to 30% cases of LCIS will subsequently develop invasive carcinoma, a risk 8-10 times higher than the control population [36]. Hence, LCIS has a definite malignant potential which could explain the decreased stromal CD34 expression observed in our study.
Malignant tumors
CD34 was consistently lost in all malignant tumors in our study, irrespective of the histological type. This was similar to the findings of various researchers earlier [11-22].
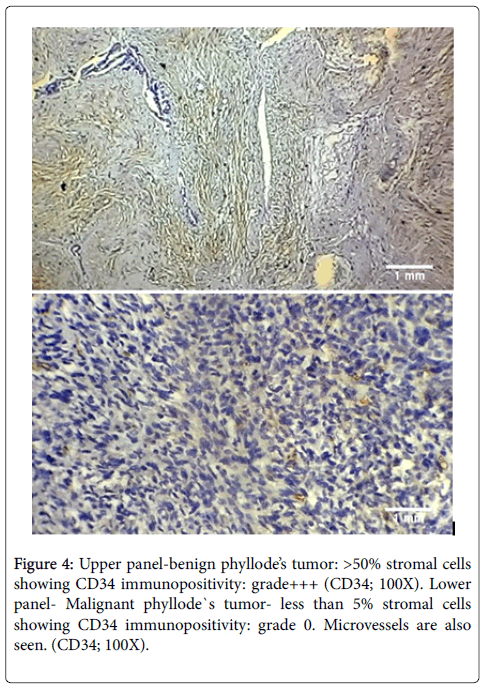
Loss of CD34 was seen in 44% cases of malignant Phyllode`s tumor (4 of 9 TDLUs) as compared to 0% cases of benign Phyllode`s (Figure 4). The difference in loss of CD34 expression between above two cases was found to be statistically significant (p<0.001).
Papillary carcinoma also showed complete loss of CD34 expression in stroma. This is in contrast to cases of papilloma, which showed grade 1+ to 2+ staining (Figure 5). The difference in loss of CD34 expression between papilloma and papillary carcinoma was also statistically significant (p<0.05).
Few cases of Paget’s disease were also studied. These were associated with DCIS or IDC. However, it was difficult to interpret the findings of stromal CD34 expression in foci of Paget`s disease. This was because Paget`s disease shows malignant cells in the epithelial lining only and the underlying TDLU are not affected by it. Our study, however, focused on determination of CD34 stromal expression around the TDLU alone. Hence, no opinion could be made on stromal CD34 expression in cases of Paget’s disease. The foci of DCIS and IDC associated with Paget’s disease were studied and showed similar stromal CD34 expression as in other cases of DCIS and IDC.
All the previous researchers in this field have shown loss of CD34 expression in cases of breast malignancies. However, there are certain controversies regarding the extent of loss of CD34 in case of IDC and ILC. While Catteau et al. and Cimpean et al. proposed that infiltrative lobular carcinoma shows less marked loss of CD34 as compared to IDC [21,22]; Kuroda et al. demonstrated similar extent of CD34 loss in both [33]. Our view on the subject is similar to that of Kuroda et al. as the frequency of loss of CD34 in cases of IDC and ILC was very similar in our study (97% loss and 96.2% loss, respectively).
To our knowledge, we were the first to describe pattern of CD34 expression in foci of metastases to axillary lymph nodes. We found that the metastatic foci showed a similar pattern of CD34 expression in the lymph nodes as those seen in the breast (Figure 6). There was loss of CD34 in 39 of 44 cases (88.6%) while rest of the cases (5 of 44) showed CD34 staining grade of 1+.
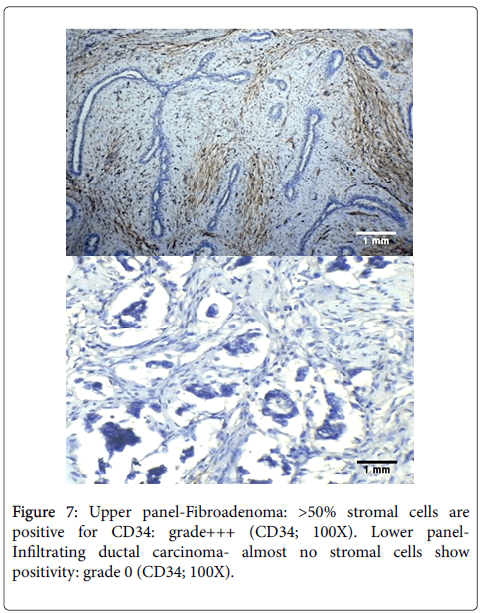
Hence, overall, most of the benign lesions in our study retained CD34 expression in their stroma while there was almost complete loss of CD34 positive stromal cells in all invasive carcinomas. An example is shown in Figure 7 demonstrating difference in staining between fibroadenoma and infiltrating ductal carcinoma. The difference in loss of CD34 expression between benign and malignant lesions overall, is highly significant statistically (p<0.001). Therefore, CD34 can be used to differentiate between benign and malignant lesions of the breast, wherein retained or diffuse CD34 expression in the stroma would suggest a benign lesion while loss of stromal CD34 cells would indicate a diagnosis of malignancy.
The reasons for loss of CD34 stromal cells in malignant lesions remain unknown. Catteau et al. proposed that several mechanisms may explain the promotion of tumor invasion that induces the transformation of CD34 fibrocytes to SMA myofibroblasts. First, CD34 fibrocytes are potent antigen-presenting cells capable of priming naive T-cells, and might be involved in specific immune surveillance. Second, CD34 fibrocytes are involved in the remodeling of stromal tissue damage not only via tissue contractility via TGF-beta, collagen I and III synthesis and SMA, but also in terms of migration factors within the injured tissue via CCR7, CXCR4, SLC, and CXCL12. Third, CD34 fibrocytes also play a role in angiogenesis via bFGF, VEGF, PDGF-a, IL-8, and MMP-9. The stromal reaction induced by carcinomatous lesions leads to acquisition of SMA expression and in turn to stabilization of the lesion (wound contraction) that helps prevent the spread of tissue damage. This may reflect a defense mechanism against ‘‘stromal invasion’’ that induces a phenomenon of stromal healing and stabilization. However, the phenotypic transformation of CD34 fibrocytes into SMA myofibroblasts could also cause the loss of most essential functions (including immunity, cell adhesion, motility, stromal remodeling and angiogenesis inhibition), and in a paradoxical manner promote tumorigenesis, thus facilitating invasion and metastatic dissemination of tumor cells [21].
Relation of stromal CD34 staining with clinicopathological parameters
In our study no significant correlation could be found between CD34 staining and either of the clinicopathological parameters, i.e., histological grade or lymph node metastasis. This was in contrast to the findings of Yazhou et al. who compared the clinicopathological parameters between invasive ductal carcinomas with and without stromal myofibroblasts and revealed significant differences in lymph node metastasis and grade of differentiation [37]. Our findings were however similar to those of Catteau et al. who also did not find any difference in CD34 expression between different histological grades of IDC [21].
Conclusion
The conclusions drawn from our study are as follows:
• Besides being expressed in the wall of the blood vessels, diffuse expression of CD34 is also found in the normal breast stroma.
• The expression of CD34 in stroma is retained in most of the benign breast lesions. Some exceptions include high grade DCIS, LCIS and fibroadenoma with atypical changes.
• The expression of CD34 in stroma is almost completely lost in all malignant neoplasms, irrespective of histological type. Metastatic foci of breast carcinoma in lymph nodes also show loss of CD34 expression in stroma.
• Loss of CD34 is related to invasive potential of the tumor. Owing to the significant differences in CD34 expression between benign and malignant breast lesions, it can potentially be used to differentiate between the two and can serve as an important diagnostic marker. Further studies can also be undertaken to establish its role as a therapeutic target in cases of breast cancer.
References
- Ronnov-Jessen L, Peterson OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76: 69-125.
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, et al. (1997) Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A 94: 6536-6540.
- Silberstein GB (2001) Tumor-stromal interactions: role of the stroma in mammary development. Breast Cancer Res 3: 218-223.
- Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR (2002) Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma xenograft model. Cancer Res 62: 3298-3307.
- Shekhar MPV, Werdell J, Santner SJ, Pauley RJ, Tait L (2001) Breast stroma plays a major role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer 61: 1320-1326.
- Adams M, Jones JL, Walker RA, Pringle JH, Bell SC (2002) Changes in Tenascin-C isoform expression in invasive and pre-invasive breast disease. Cancer Res 62: 3289-3297.
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P (1990) A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 348: 699-704.
- Jones JL, Glynn P, Walker RA (1999) Expression of MMP-2 and MMP-9, their inhibitors and the activator MT1-MMP in primary breast carcinomas. J Pathol 189: 161-168.
- Chesney J, Bacher M, Bender A, Bucala R (1997) The peripheral blood fibrocyte is a potent antigen presenting cell capable of priming naïve T cells in situ. Proc Natl Acad Sci U S A 94: 6307-6312.
- Wessel C, Westhoff CC, Nowak K, Moll I, Barth PJ (2008) CD34 (+) fibrocytes in melanocytic nevi and malignant melanomas of the skin. Virchows Arch 453: 485-489.
- Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) Virchows Arch 440: 298-303.
- Van de Rijn M, Rouse RV (1994) CD34 A review. Appl Immunohistochem 2: 71-80.
- Yamazaki K, Eyden BP (1995) Ultrastructural and immunohistochemical observations on intralobular fibroblasts of human breast with observations on the CD34 antigen. J Submicrosc Cytol Pathol 27: 309-323.
- Moore T, Lee AHS (2001) Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology 38: 62-76.
- Silverman JS, Tamsen A (1996) Mammary fibroadenomas and some phyllodes tumour stroma are composed of CD34+ fibroblasts and factor XIIIa+ dendrophages. Histopathology 29: 411-419.
- Chen CM, Chen CJ, Chang CL, Shyu JS, Hsieh HF, et al. (2000) CD34, CD117 and actin expression in phyllodes tumour of the breast. J Surg Res 94: 84-91.
- Goldblum JR, Reith JD, Weiss SW (2000) Sarcomas arising in dermatofibrosarcoma protruberans: a reappraisal of biologic behaviour in eighteen cases treated by wide local excision with extended clinical follow up. Am J Surg Pathol 24: 1125-1130.
- Kirchmann TT, Prieto VG, Smoller BR (1995) Use of CD34 is assessing the relationship between stroma and tumour in desmoplastic keratinocyte neoplasms. J Cutan Pathol 22: 422–426.
- Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, et al. (2000) Differential expression of CD34 in normal colorectal tissue, peritumoural inflammatory tissue and tumour stroma. J Clin Pathol 53: 626-629.
- Chauhan H, Abraham A, Phillips JRA, Pringle JH, Walker RA, et al. (2003) There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J ClinPathol 56: 271-276.
- Catteau X, Simon P, Vanhaeverbeek M, Noel JC (2013) Variable Stromal Periductular Expression of CD34 and Smooth Muscle Actin (SMA) in Intraductal carcinoma of the Breast. PLoS ONE 8: e57773.
- Cimpean AM, Raica M, Narita D (2005) Diagnostic significance of the immunoexpression of CD34 and smooth muscle cell actin in benign and malignant tumors of the breast. Rom J Morphol Embryol 46: 123-129.
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol med 1: 71-81.
- Papadas T, Batistatou A, Ravazoula P, Zolota V, Goumas P (2001) S-100 protein-positive dendritic cells and CD34-positive dendritic interstitial cells in palatine tonsils. Eur Arch Otorhinolaryngol 258: 243-245.
- Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramswamy A (2002) CD34 (+) fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 440: 128-133.
- Barth PJ, Ramswamy A, Moll R (2002) CD34 (+) fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch 441: 564-568
- Kuroda N, Miyazaki E, Hayashi Y, Toi M, Hiroi M, et al. (2004) The disappearance of CD34-positive and alpha-smooth muscle actin-positive stromal cells associated with human intrauterine and tubal pregnancies. Histol Histopathol 19: 707-713.
- Kuroda N, Nakayama H, Miyazaki E, Hayashi Y, Toi M, et al. (2005) Distribution and role of CD34-positive stromal cells and myofibroblasts in human normal testicular stroma. Histol Histopathol 743-751.
- Kuroda N, Toi M, Nakayama H, Miyazaki E, Yamamoto M, et al. (2004) The distribution and role of myofibroblasts and CD34-positive stromal cells in normal pancreas and various pancreatic lesions. Histol Histopathol 19: 59-67.
- Nakayama H, Enzan H, Yamamoto M, Miyazaki E, Hidaka C, et al. (2003) CD34-positive stromal cells in primary lung carcinomas. Oncol Rep 10: 1213-1316.
- Nakayama H, Naruse K, Miyazaki E, Hiroi M, Kiyoku H, et al. (1999) CD34-positive cells in breast lesions and Enzan H. The specific distribution of dendritic interstitial cells at the tumor border of major salivary gland pleomorphic adenomas. Mod Pathol 12: 445-449.
- Ramaswamy A, Moll R, Barth P (2003) CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 443: 536-540.
- Kuroda N, Jin YL, Hamauzu T, Toi M, Miyazaki E, et al. (2005) Consistent lack of CD34-positive stromal cells in the stroma of malignant breast lesions. Histol Histopathol 20: 707-712
- Lewis JT, Hartmann LC, Vierkant RA, Maloney SD, Shane Pankratz V, et al. (2006) An analysis of breast cancer risk in women with single, multiple and atypical papilloma. Am J Surg Pathol 30: 665-672.
- Eusebi V, Foschini MP, Cook MG, Berrino F, Azzopardi JG (1989) Long term follow up of in situ carcinoma carcinoma of the breast with special emphasis on clinging carcinoma. Semin Diagn Pathol 6: 165-173.
- Rosai J (2011) Rosai and Ackerman`s Surgical Pathology, 10th ed. Elsevier Inc, pp: 1665.
- Yazhou C, Wenlv S, Weidong Z, Licun W (2004) Clinicopathological significance of stromal myofibroblasts in invasive ductal carcinoma of the breast. Tumor Biol 25: 290-295.
Citation: Khan AA, Alam K, Harris H (2017) A Clinicopathological Study of cd34 Antigen Expression in Benign and Malignant Breast Lesions. J Clin Exp Pathol 7:321. DOI: 10.4172/2161-0681.1000321
Copyright: © 2017 Khan AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6224
- [From(publication date): 0-2017 - Jul 19, 2025]
- Breakdown by view type
- HTML page views: 5122
- PDF downloads: 1102