A Case Study of the Spread of the Invasive Urchin Diadema setosum (Leske, 1778) in the Eastern Mediterranean (Syria)
Received: 11-Dec-2022 / Manuscript No. JFLP-22-83088 / Editor assigned: 13-Dec-2022 / PreQC No. JFLP-22-83088(PQ) / Reviewed: 27-Dec-2022 / QC No. JFLP-22-83088 / Revised: 02-Jan-2023 / Manuscript No. JFLP-22-83088(R) / Accepted Date: 04-Jan-2023 / Published Date: 09-Jan-2023 DOI: 10.4172/2332-2608.1000384
Abstract
During the past three years: 2019, 2020 and 2021, the presence of more invasive alien species has been detected in the Syrian coast, the needle-spined urchin (Diadema setosum) (long-spined sea urchin, black long-spine sea urchin) may be among the worst. It appears in high densities and large sizes at depths of 8-10 m in the northern sector of the Syrian coast since 2016.
This is the first record of this species in Syria, thus, the map of documenting his presence in the eastern Mediterranean is completed.
Keywords
Diadematidae; Needle-spined sea urchin; Non-indigenous species; Invasive species; Syrian coast
Introduction
The geographical location of the Syrian coast in the central part of the eastern, Mediterranean and its closeness to the Suez Canal, has helped many alien species of Indian, Pacific and East Atlantic oceanic origins to invading this region and dominate within the local benthic communities after the opening of the Suez Canal in 1869 by Lyspian migrantion from the Red Sea. The results of studies in Syria during the past few years have shown a steady increase in the number of alien species, as is the case in other countries of the region [1, 2]. The percentage of alien species reached until 2021 (15.73%) of the total number of documented species on the Syrian coast, invasive species consists (37.29%) of the total aliens [3].
Certainly, there are additional factors that have greatly contributed to the increase of these species, the most important are: the balance water in terms of temperature and salinity, sea transport and the fouling, and this explains the presence of the largest number of alien species and the recording many of them for the first time in the Syrian marinas and ports [3].
The hydrological and geological characteristics features of the Syrian coast help in the stability of alien species and their alteration into invasive species with the passage of time. Overfishing, pollution, and the absence of any plan to face up to these species may have a role in the expansion of their spread. It can be said that the Syrian marine environment, with its living components and physical properties, has become a tropical environment, like many areas in the region.
The current study documents the presence of the needle-spined urchin for the first time in Syria. It is a tropical species of sea urchin that spreads on a large scale in the world, and forms large population; it has a very important role in the environment as an herbivorous organism [3, 4].
This species is widely distributed throughout the Indian and Pacific Oceans, east to the Australian coast, it appeared in the Persian Gulf and the Red Sea [4, 5], and invaded the Mediterranean Sea since 2006 through the Suez Canal [6]. Its presence was recorded for the first time in Turkey [6], and it is now widespread along the Turkish coasts, in the Aegean Sea [7], and south towards the eastern Mediterranean [7, 8]. It was recorded in Greece in 2020 [9], recently, few specimens were observed in the southern coast of Mediterranean (Egypt and Libya) [4, 10].
The needle-spined urchin lives in shallow water at depths ranging from 1 - 70 m, and is often found at a depth of 4-6 m [11]. It prefers rocky substrates and coral reefs, and hides in rocky crevices, especially in intense light, and can also be found on sandy bottoms and sea grass meadows [4].
This species has an important environmental role as it works to maintain the integrity of coral reefs by scraping and feeding on algae, thus preventing algae from dominating the vital ecosystem of coral reefs [12].
Materials and Method
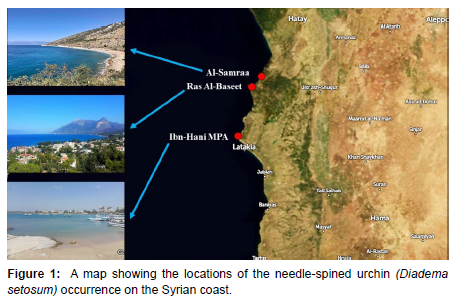
For the first time, three individuals of the needle-spined urchin were collected by hand from shallow waters of a rocky substrate covered with seaweed at a depth of 0.5 – 6 m from Ibn – Hani marine protect area next to the Higher Institute of Marine Research (35°35’33.0”N 35°44’31.9”E) at the north of Latakia on 7 October-2020. Then, twenty individuals of the needle-spined urchin were collected by free diving on 13 January-2021 from the same area, large numbers were observed in deeper areas and the size of individuals was large. A massive spread of this species was observed in Al-Bassit marina in 2021 and in Al-Samra site northern Syria (Figure 1).
All volumetric (total wet weight) and size measurements (diameter and height of the test, length of spines) of the collected specimens were made in the laboratory, as they were photographed before and after removing the spines.
Results
Classification
The needle-spined urchin belongs to the family Diadematidae, of the phylum Echinodermata, class Echinoidea, and order Diadematoida [13].
Kingdom: Animalia
Phylum: Echinodermata
Class: Echinoidea
Order: Diadematoida
Family: Diadematidae
Genus: Diadema
Species: Diadema setosum (Leske, 1778).
Description of the needle-spined urchin (Diadema setosum) Specimens
External appearance: The needle-spined urchin grows to about 80 mm in diameter, and it has a spherical or disc-shaped body, slightly compressed dorsally ventrally, and the body is generally black in color (Figures 2a, 2b).
Five white spots on the inter ambulacral regions in the test (Figure 2a) as well as a bright orange ring around the tip of the anal cone are clear (Figures 2b, 2c) [12, 14]. It also shows blue lines along its test (Figure 2d).
Measurements of the studied the needle-spined urchin specimens is listed in Table 1. The size of the individuals ranged between (4.58– 7.50 cm) for diameter and its height ranged between (2.33-3.70 cm). The length of spines ranged between (7- 30 cm) and the specimens wet weight ranged between (80.75–390 g.).
| Characteristic | Rang |
|---|---|
| Test Diameter (TD) | 4.58 – 7.50 cm. |
| Test Height (TH) | 2.33 – 3.70 cm. |
| Length of spines | 7 – 30 cm |
| Specimen Wet Weight (SW) | 80.75 – 390 g. |
Table 1: Diameter, height of the test, length of spines and total wet weight of the studied needle-spined urchin (Diadema setosum).
The genus Diadema differs from other genera by its tube feet in tripartite groups, tubular pedicellaria of the needle-spined urchin being triangular but broad and spoon-like [14].
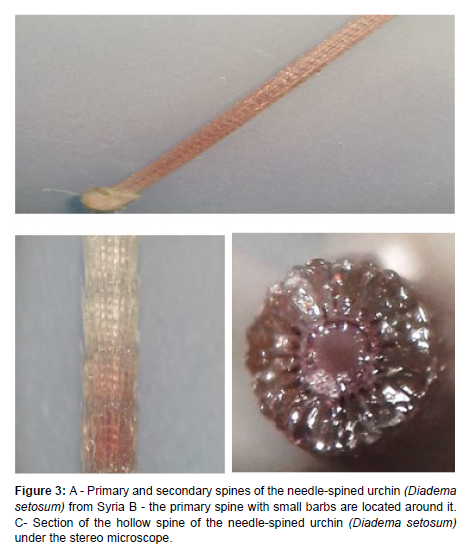
Spines
The spines of the specimens were often black, or purple or banded dark brown in other smaller specimens, there are two types of spines: primary and secondary. The primary spines are longer and thicker than the secondary (Figure 3A), it is brittle and long, up to 30 cm in length in large adults, and is pointed and sharp at the end. The outer surface of the primary spines is surrounded by very small barbs, which are directed towards the head of the primary spine [14] (Figure 3B). These spines are round, hollow and contain a slight poison (Figure 3C). The secondary (small) spines are distributed around each primary long spine and originate from the body of the urchin. The base of the primary spines is inflated (2 mm in size) at the point of contact with the body of the urchin [15].
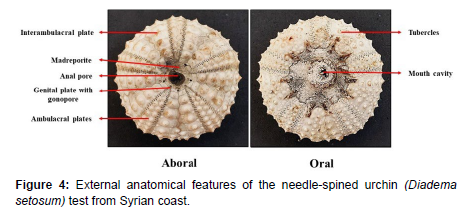
Test
The body of the needle-spined urchin is mainly divided into oral and aboral halve, with radially visible organs around the central pore opposite the oral surface and containing the mouth, while the aboral surface containing the anus. Radially, the body is divided into 10 sections, alternating between ambulacral and interambulacral regions, each consisting of two skeletal plates. The ambulacral regions carry flexible tube feet that stand out from the foot holes, while the interambulacral regions do not bear tube feet.
The mouth is a large opening in the center of the oral surface of the test, surrounded by a flexible membrane this membrane bears buccal podia (short tube feet) and branched nostrils.
At the opposite end, the anus is surrounded by a circular membrane called the periproct. It is bordered by 10 plates, 5 of which are reproductive plates, containing gonospores that release gametes, and 5 is perforated through which the entry of sea water is controlled in the aquatic blood vessels system, called the refinery [14] (Figure 4).
Currently, the presence of this species is abundant in some areas in northern Syria, and information from some divers indicated that they noticed the presence of some individuals of this species since 2016 in the same locations.
The presence of this species in association with the similar species (Diadema antillarum), which differs from it by the thickness of the spines, the absence of the red ring around the anus, and the decoration of the test. Ammar molecular analyses should help to identify the differences more clearly, and this is what will be worked on later [16].
From the other side, the high abundance of this species in the studied area indicates the appropriateness of the conditions of the marine environment in Syria and the eastern Mediterranean for its growth and reproduction, especially some hydrological conditions such as temperature and salinity.
The temperature of the subsurface water during 2020-2021 in Ibn-Hani MPA had ranged between (16.9-30.8°C), the dissolved Oxygen concentration was about (7.5 mg/L), and the salinity ranged between (36.4-38.7‰). These measurements seem appropriate for its reproduction, as it is assumed that it reproduces in the summer months, where the formation of gametes starts in April and May when the water temperature rises above 25°C, and ovulation occurs among June and September [6, 12].
Thus, the environmental changes and the increase in the temperature of the eastern of Mediterranean as a result of climatic changes [17], may be one of the reasons for the success of this species in adapting and establishing along the Syrian Coast in addition to the calcareous and coralligenous constructions [16, 18], by transition from the Indian and Pacific Oceans through the Suez Canal towards the Mediterranean Sea, as well as the availability of an algal cover suitable for this urchin to feed on.
Urchin nowadays is included in the list of the worst invasive alien species in the eastern Mediterranean [19]. Its numbers in the region are increasing day by day, especially in northern Syria. It seems that the hydrographic conditions of the Syrian coast are very suitable for the growth and spread of this species with other invasive species of algae, fish and invertebrates.
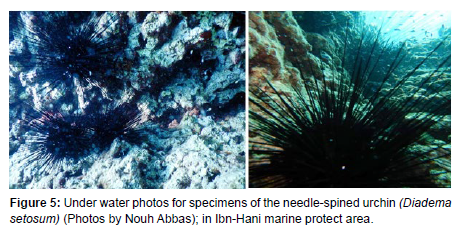
The geomorphological characteristics of the site may be the most important factor in the distribution of the species in Syria, and it seems that this species prefers quiet places and bays away from the influence of human activities, particularly hunting. This is clear from the photos and sites in which his presence was monitored in abundance, reaching up to 7 individuals/m2 (Figure 5).
The bottom in these tow sits consists of bio constructions and coralliferous formations interspersed with many grooves as is evident in the (Figure 5), while the benthic community is dominated by invasive alien species of fish such as lionfish, puffer, balloon, crabs such as Portunus signs, molluscs such as Pinctada imbricata radiata, Chama pacifica, Spondylus multimuricatus, Ircinia sp, and invasive algae such as Asparaguses taxiformis and few species of crustose and coralline algae [3].
Documenting the presence of this species on the Syrian coast and photographing it by one of the amateur divers before we noticed it as scientists and researchers indicates the importance and role of these citizens in discovering the presence of any new alien species in the local environment, which reduces the period between its entry, discovery and documentation.
With this documentation, the map of the distribution of this invasive species in the eastern Mediterranean has been completed, and it is necessary to monitor its expanded and spread in the southern part of the Syrian coast, as it is still absent.
The economic importance of the needle-spined urchin beings positive when the citizens use to eat this species which is eaten more specifically than any other urchin in the world [12]. It is can also be used in medicine, it has the ability to develop a new type of antibiotic in the pharmaceutical field and is rich in bioactive compounds that have a function as antioxidants, anti-inflammatory, tumors, cancer and bacteria.
The negative impacts concerns with the long and thin spines witch cause painful injuries to swimmers, divers, and fishermen when a person steps on or falls on. The sharp and pointed ends of the primary spines can pierce the skin, break and remain embedded in the flesh, releasing poison from its tissues and cavities. The poison causes redness, swelling and severe pain that subsides after a few hours [20].
Finally, we say that the spread of poisonous sea urchins of the species (Diadema setosum) and (Diadema antillarum) in the coastal area, where tourism and recreation are active, requires taking certain procedures to get rid of them. It also more research is required, especially studying the biochemical composition and studying the properties of these toxins against some chronic diseases.
Acknowledgement
Many thanks to Mr Nouh Abbas who photographed the underwater samples as well as to the anonymous reviewers who reviewed and corrected this article scientifically and linguistically.
References
- Mavruk S, Bengil F, Yeldan H, Manasirli M, Avsar D (2017) The trend of lessepsian fish populations with an emphasis on temperature variations in Iskenderun Bay, the Northeastern Mediterranean. Fish Oceanogr 26: 542-554.
- Zenetos A, Galanidi M (2020) Mediterranean non-indigenous species at the start of the 2020s: recent changes. Mar Biodivers Rec 13: 10.
- Ammar I, Arraj H, Dib F, Arabia I (2022) Updated list of alien macrozoobenthic species along the Syrian coast. Int J Aquat Biol 10: 2023.
- Vafidis D, Antoniadou C, Voulgaris K, Varkoulis A, Apostologamvrou C (2021) Abundance and population characteristics of the invasive sea urchin Diadema setosum (Leske 1778) in the south Aegean Sea (eastern Mediterranean). JBRT 28: 11.
- Bronstein O, Georgopoulou E, Kroh A (2017) On the distribution of the invasive long-spined echinoid Diadema setosum and its expansion in the Mediterranean Sea. Mar Ecol Prog Ser 583: 163-178
- Yokes B, Galil BS (2006) The first record of the needle-spined urchin Diadema setosum (Leske 1778) (Echinodermata: Echinoidea: Diadematidae) from the Mediterranean Sea-Aquatic Invasions. Journal compilation 188-190.
- Mytilineou C, Akel E, Babali N, Balistreri P, Bariche M et al (2016) New Mediterranean Biodiversity Records (November, 2016).Mediterr Mar Sci 17: 794-821.
- Galanos CJ, Kritikos S (2019) Diadema setosum (Leske, 1778) (Echinodermata, Echinoidea, Diadematidae), first record for Simi island, Hellas, eastern Mediterranean.Parnassiana Archives 7: 15-19.
- Pirkenseer CM (2020) Alien species in southern Laconia, Kythira Island and southern Messenia (Greece): new and additional records and updated record maps.J Black Sea/Mediterranean Env 26: 145-175.
- Nour OM, Al Mabruk SAA, Adel M, Corsini-Foka M, Zava B, et al (2022) First occurrence of the needle-spined urchin Diadema setosum (Leske 1778) (Echinodermata, Diadematidae) in the southern Mediterranean Sea.BioInvasions Records 11: 199-205.
- Palomares MLD, Pauly D (2019) Diadema setosum (Leske, 1778) porcupine sea urchin. Sea Life Base.
- Muthiga NA, McClanahan TR (2020) Diadema. Sea urchins: biology and ecology Elsevier 397-418
- WoRMS Editorial Board (2022) World Register of Marine Species.
- Lessios HA, Kessing BD, Pearse JS (2001) Population structure and speciation in tropical seas: Global phylogeography of the sea urchin Diadema. Int J Org Evol 55:955-975.
- Schultz H (2006) Sea urchins: A guide to worldwide shallow water species Third edition Heinke & Peter Schultz Partner Scientific Publications: Hemdingen 484.
- Ammar IA (2021) First Record and Massive Spread of Tropical Long-Spined Sea Urchin Diadema antillarum in the Mediterranean Sea (Syrian Coast). JEES 3: 1-4.
- Pisano A, Marullo S, Artale V, Falcini F, Yang C, et al. (2020) New evidence of Mediterranean Climate change and variability from sea surface temperature observations. Rem Sens 12: 132.
- Ingrosso G, Abbiati M, Badalamenti F, Bavestrello G, Belmonte G, et al (2018) Mediterranean Bioconstructions Along the Italian Coast. Adv Mar Biol 79: 61-136
- Bedry R, De Haro L, Bentur Y, Senechal N, Galil BS (2021) Toxicological risks on the human health of populations living around the Mediterranean Sea linked to the invasion of non-indigenous marine species from the Red Sea: A review. Toxicon 191:69-82
- Williamson JA, Fenner PJ, Burnett JW, Rifkin JF (1996) Venomous and poisonous marine animals: a medical and biological handbook. University of New South Wales Press, 504.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Ammar IA, Barhoum YM (2023) A Case Study of the Spread of the Invasive Urchin Diadema setosum (Leske, 1778) in the Eastern Mediterranean (Syria). J Fisheries Livest Prod 11: 384. DOI: 10.4172/2332-2608.1000384
Copyright: © 2023 Ammar IA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2048
- [From(publication date): 0-2023 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 1601
- PDF downloads: 447