A Case Report of Lynch-Like Syndrome Diagnosed due to Intussusception Treated with Laparoscopic Colectomy
Received: 02-Apr-2024 / Manuscript No. DPO-24-131722 / Editor assigned: 05-Apr-2024 / PreQC No. DPO-24-131722 (PQ) / Reviewed: 19-Apr-2024 / QC No. DPO-24- 131722 / Revised: 26-Apr-2024 / Manuscript No. DPO-24- 131722 (R) / Published Date: 03-May-2024 DOI: 10.4172/2476-2024.9.2.230
Abstract
Background: Lynch syndrome, caused by germline mutations in mismatch repair proteins, can cause early onset of colorectal cancer. Lynch-like syndrome was recently defined as a third type of microsatellite instability tumor differ to Lynch syndrome and sporadic MSI colorectal cancer without either a germline variant of mismatch repair genes or hypermethylation of the MLH1 gene. Here, we report a rare case of Lynch-like syndrome diagnosed due to intussusception.
Case presentation: A 28-year-old man presented with right lower abdominal pain for approximately 3 months and had defecated black stool for several weeks. Computed tomography revealed a contrast-enhanced tumor and lymph nodes with a crab-claw-like image extending into the ascending colon. Colonoscopy revealed a large submucosal tumor-like lesion with ulceration. Laparoscopy-assisted ileocecal resection with level three lymph node dissections was performed 3 days after endoscopic reduction of the intussusception. Histological diagnosis was poorly differentiated adenocarcinoma with mucus-producing component. Immunohistochemistry demonstrated no MutL Homolog 1 (MLH1) or Postmeiotic Segregation Increased 2 (PMS2) expression in the tumor. Genetic analysis revealed high microsatellite instability, and no BRAF mutations, no MLH1 promoter methylation, and no MLH1 epimutation was found. Although these findings suggested Lynch syndrome, further genetic analysis revealed no pathological mutations of MLH1 and PMS2 in germline and somatic DNA and RNA. Together these findings, the patient was thought as Lynch-like syndrome. A novel outstanding mechanism may have caused defective mismatch repair in the tissue of this young patient, which should be studied in the future.
Conclusion: We encountered a rare case of Lynch-like syndrome in which the diagnosis was due to intussusception. There might be a novel outstanding mechanism resulting in dMMR in the tissue of such a young patient.
Keywords: Case report; Lynch syndrome; Microsatellite instability; Juvenile colon cancer; Intussusception
Abbreviations
dMMR: Defective Mismatch Repair; MSI: Microsatellite Instability; MLH1: Mutl Homolog 1; MSH2: Muts Homolog 2; PMS2: Postmeiotic Segregation Increased 2; MSH6: Muts Homolog 6; BRAF: V-Raf Murine Sarcoma Viral Oncogene Homolog B1; CT: Computed Tomography
Introduction
Intussusception is relatively rare in adults, accounting for approximately 5% of all intussusception cases. Cancer is the most common cause of intussusception, occurring in the colon in about 60% of cases [1]. Lynch syndrome is an autosomal dominant disorder caused by germline mutations in a gene cluster that encodes Mismatch Repair (MMR) proteins [2]. When the MMR function is defective (dMMR), errors made during DNA replication are not repaired and may accumulate, leading to cancer. This phenomenon is known as Microsatellite Instability (MSI). Therefore, MSI testing for malignant tumors and immune histochemical testing for MMR proteins may be helpful for diagnosing Lynch syndrome. Lynch syndrome is diagnosed by confirming that the Amsterdam and Bethesda criteria [3] are met (primary screening), followed by MSI or immunohistological testing (secondary screening) [4,5]. If abnormalities are detected in secondary screening, germline genetic testing for the MMR genes, such as MLH1, MSH2, PMS2, or MSH6 is performed; the detection of pathological mutations is the basis for a definitive diagnosis of Lynch syndrome [6]. Lynch-like syndrome was recently defined as a third type of microsatellite instability tumor differ to Lynch syndrome and sporadic MSI colorectal cancer without either a germline variant of mismatch repair genes or hypermethylation of the MLH1 gene [7,8].
We encountered a case of colorectal cancer in a 28-year-old patient. Genetic analysis strongly suggested Lynch syndrome because of the MSI-high and BRAF mutation-negative results. Here, we report a rare case of Lynch-like syndrome diagnosed due to intussusception with further analysis of germline and somatic DNA and RNA.
Case Presentation
The patient, who visited the Gastroenterology Center of Shunan Memorial Hospital, was a 28-year-old man who had been experiencing right lower abdominal pain for approximately 3 months. He had also defecated black stools for several weeks. His medical history included gastric ulcers during his teens. No significant family history of cancer, including colorectal cancer, was noted. A physical examination revealed tenderness and rebound pain in the right lower abdomen. Blood tests showed no liver or renal function problems and a microcytic iron deficiency anemia. His hemoglobin and serum Fe levels were 10.4 g/dL and 15 g/dL, respectively. Tumor markers were analyzed but soluble Interleukin-2 Receptor (sIL-2R), Carcinoembryonic Antigen (CEA), and Carbohydrate Antigen 19-9 (CA19-9) levels were within normal ranges.
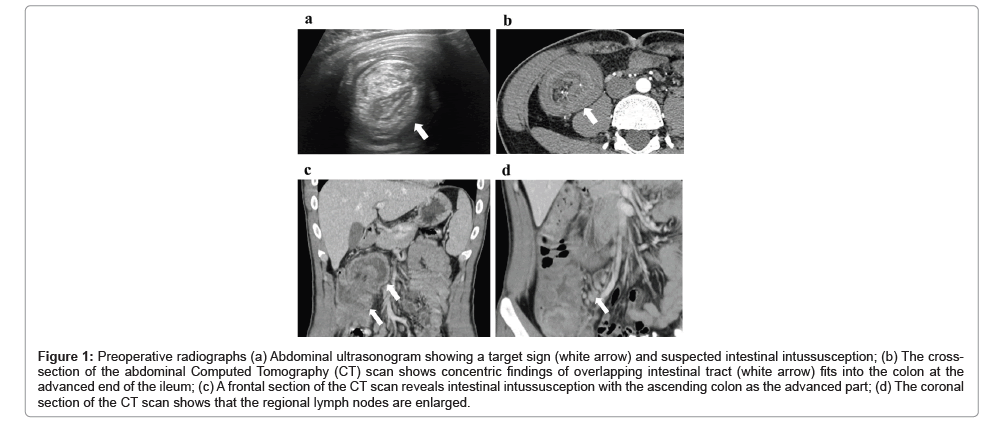
Abdominal ultrasonography showed that the target sign was superimposed on the ascending colon to the transverse colon at the hepatic flexure (Figure 1a). Computed tomography (CT) revealed concentric circles of invagination in the intestine fitting into the ascending colon (Figure 1b), with the terminal portion of the ileum as the advanced portion (Figure 1c). The regional lymph nodes were also enlarged (Figure 1d).
Figure 1: Preoperative radiographs (a) Abdominal ultrasonogram showing a target sign (white arrow) and suspected intestinal intussusception; (b) The cross- section of the abdominal Computed Tomography (CT) scan shows concentric findings of overlapping intestinal tract (white arrow) fits into the colon at the advanced end of the ileum; (c) A frontal section of the CT scan reveals intestinal intussusception with the ascending colon as the advanced part; (d) The coronal section of the CT scan shows that the regional lymph nodes are enlarged.
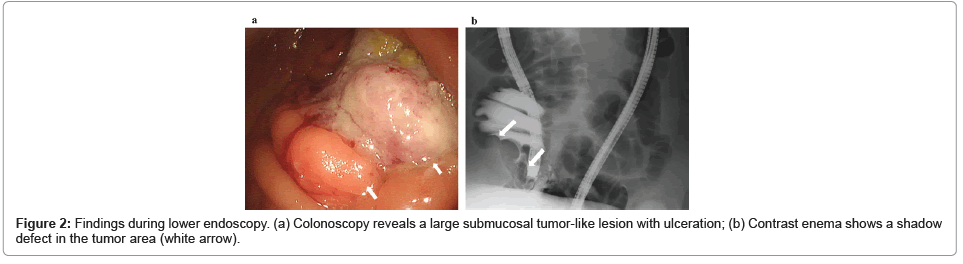
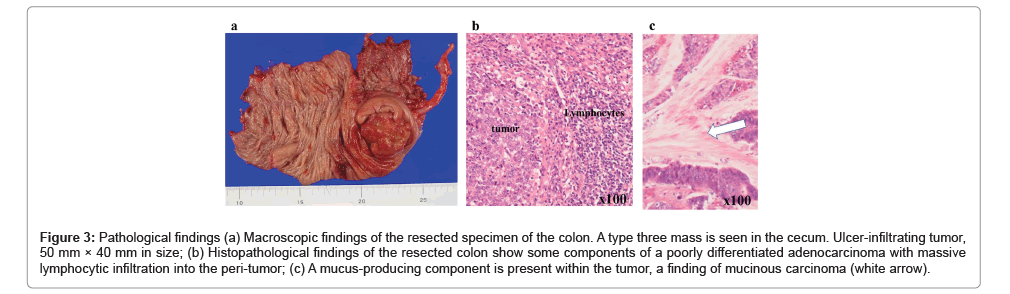
An emergency colonoscopy was performed for diagnosis, which resulted in the reduction of the invagination and revealed a large submucosal tumor-like lesion with ulceration in the cecum (Figure 2a). Pathological finding of biopsy was adenocarcinoma. Contrast enema revealed a shadow defect in the cecum (Figure 2b). Based on these results, colon cancer was considered in the differential diagnosis. Laparoscopic ileocecal resection with D3 lymph node dissection was performed, and the patient was discharged postoperatively. The tumor was type three with a size of 50 mm × 40 mm (Figure 3a). Pathological findings showed that it was mainly an intermediately differentiated adenocarcinoma with some components of poorly differentiated adenocarcinoma, with infiltrating lymphocytes (Figure 3b), and with mucus-producing component within the tumor, which is a characteristic of Lynch syndrome derived colon cancer (Figure 3c). The tumor invaded into the subserosa (pT3), had no evidence of lymph node metastasis (pN0) or distant metastasis (M0), and pathological stage was Stage II (UICC8th edition). Genetic analysis of the tumor revealed a v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation (G13D), wild-type BRAF, and MSI-high. This case of juvenile colon cancer, with no lymph node metastasis, wild-type BRAF, MSI-high, and some components of poorly differentiated adenocarcinoma in the histological findings, strongly suggested Lynch syndrome.
Figure 3: Pathological findings (a) Macroscopic findings of the resected specimen of the colon. A type three mass is seen in the cecum. Ulcer-infiltrating tumor, 50 mm × 40 mm in size; (b) Histopathological findings of the resected colon show some components of a poorly differentiated adenocarcinoma with massive lymphocytic infiltration into the peri-tumor; (c) A mucus-producing component is present within the tumor, a finding of mucinous carcinoma (white arrow).
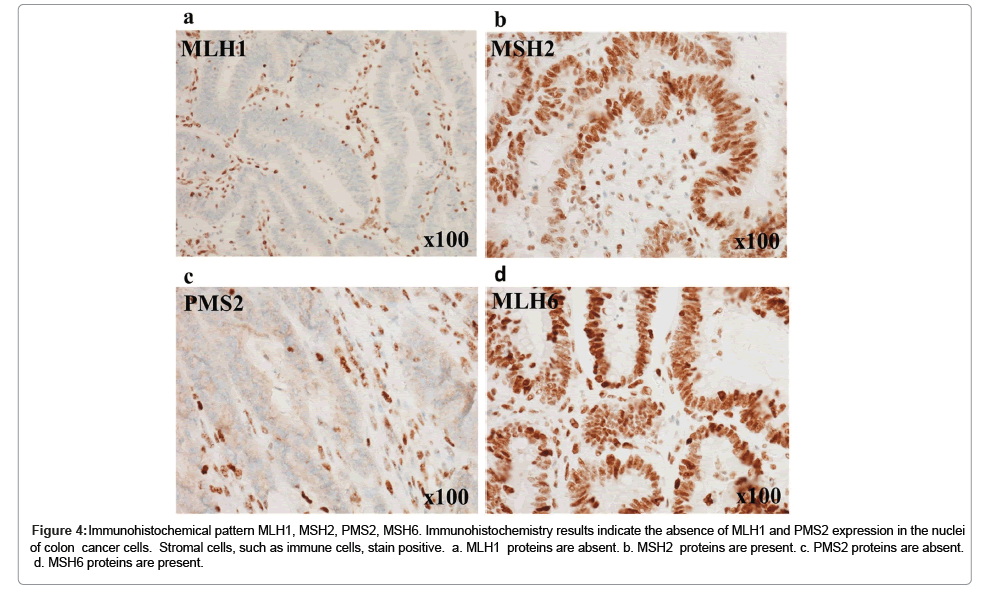
Figure 4: Immunohistochemical pattern MLH1, MSH2, PMS2, MSH6. Immunohistochemistry results indicate the absence of MLH1 and PMS2 expression in the nuclei of colon cancer cells. Stromal cells, such as immune cells, stain positive. a. MLH1 proteins are absent. b. MSH2 proteins are present. c. PMS2 proteins are absent. d. MSH6 proteins are present.
Immunohistochemistry was performed to examine the protein expression of MLH1, MSH2, PMS2, and MSH6. Sections were incubated with anti-MLH1 antibody (mouse monoclonal antibody, M1, Roche Diagnostics, Tokyo, Japan), anti-MSH2 antibody (mouse monoclonal antibody, G219-1129, Roche Diagnostics), anti-PMS2 antibody (mouse monoclonal antibody, A16-4, Roche Diagnostics), and anti-MSH6 antibody (rabbit monoclonal antibody, SP93, Roche Diagnostics). Immunohistochemistry revealed the absence of MLH1 and PMS2 in the nuclei of the colon cancer cells (Figures 4a-4d).
Whole Exome Sequencing (WES) was performed using DNA from peripheral blood and the tumor, as previously described [9]. WES revealed germline mutations in PMS2 (rs2228006, c.1621A>G), PMS2 (rs1805321, c.1408C>T), and MSH2 (rs63750716, c.505A>G), which were not pathogenic. The somatic mutation in MSH6 (NM_000179.2|c. 2780T>C) was also not pathogenic. Another possibility for no expression of MLH1 and PMS2 is MLH1 promoter methylation or MLH1 epimutation as the underlying pathogenesis in this case [10]. Therefore, we analyzed the tumor genes; however, no MLH1 promoter methylation or MLH1 epimutation was found.
Finally, we performed expressional analysis of peripheral blood mononuclear cells for all coding regions of MLH1, MSH2, MSH6, and PMS2 using RT-PCR and DNA sequencing. There is no variant of these five genes was found, suggesting the patient had a normal mismatch repair mechanism.
These analyses were conducted according to the Declaration of Helsinki and approved by the Institutional Ethics Review Boards of Shunan Memorial Hospital (R04-07) and Yamaguchi University (approval no. H17-83). Written informed consent was obtained from the patient for publication of this report and any accompanying analyses.
Discussion
Here, we report a rare case of Lynch-like syndrome diagnosed due to intussusception. Only three cases with the same pathological condition have been reported [11-13]. If a young person has colorectal cancer, close examination for Lynch syndrome should be performed. Although the results of the MSI-high and wild type BRAF indicated the possibility of Lynch syndrome in this juvenile colon cancer case, the family history did not meet the Amsterdam and Bethesda criteria [3]. Next, we examined the immunohistochemical patterns and exome sequences of MLH1, MSH2, PMS2, and MSH6. Immunohistochemistry indicated the absence of MLH1 and PMS2 expression in the tumors. These data suggest the loss of the MLH1 function because the expression of PMS2 depends on the MLH1 function [14]. In contrast, genetic analysis of the peripheral blood and tumor revealed no pathological mutations in MLH1, MSH2, PMS2, or MSH6. Germline mutations in MMR-related genes cannot be identified in 10%-15% of patients with clinically suspected Lynch syndrome; thus, Lynch-like syndrome has been proposed for patients with colorectal cancer who are MSI-high but do not have germline mutations in the MMR genes [7,8,15]. Moreover, no MLH1 promoter methylation or MLH1 epimutation was found [10]. Finally, we analyzed the RNA expression of peripheral blood mononuclear cells for all coding regions of MLH1, MSH2, MSH6, and PMS2. No variant or low expression of these five genes was found, suggesting that the patient has a normal mismatch repair mechanism. A new question, however, arose, “why did the patient suffer juvenile colon cancer at the age of 28 years?” There might be a novel mechanism underlying dMMR, such as a still unknown part of the gene or simply because our technical capacity, in the tissue of such a young patient.
The risk of Colorectal Cancer (CRC) was reported to lower in families with Leukemia and Lymphoma Society (LLS) than among patients with genetically confirmed LS but significantly higher than in cases of sporadic CRC [7]. This report suggests the need for a special surveillance strategy for these patients and their relatives. An intensive follow-up for such patients to identify new emerging cancers in other organs is necessary.
Conclusion
We encountered a rare case of Lynch-like syndrome in which the diagnosis was due to intussusception. Although MSI-high, wild-type BRAF, the absence of MLH1 and PMS2 expression, no MLH1 promoter methylation, and no MLH1 epimutation suggested. Lynch syndrome, no pathological mutations in the germline or somatic gene sequence were observed. There might be a novel mechanism resulting in dMMR in the tissue of such a young patient.
Declarations
Ethics approval and consent to participate
These analyses were conducted according to the Declaration of Helsinki and approved by the Institutional Ethics Review Boards of Shunan Memorial Hospital (approval no. R04-07) and Yamaguchi University (approval no. H17-83). Written informed consent was obtained from the patient for publication of this report and any accompanying analyses.
Consent for publication
Informed consent to publish the details of the case was obtained from the patient.
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported in part by JSPS KAKENHI Grant Number 21K08711 of S Hazama.
Authors’ contributions
Ryosuke Ogata , Toshiyuki Fuji, and Shoichi Hazama treated the patient. Ryosuke Ogata and Shoichi Hazama collected the clinical data and wrote the manuscript. Shoichi Hazama, Ryosuke Ogata, Keisuke Hino, Ryouichi Tsunedomi, Hiroaki Nagano,Kiwamu Akagi and Takeshi Nagasaka applied and assessed the results of the genetic examination and discussed them. Ryosuke Ogata and Shoichi Hazama applied and assessed the results of the immunohistochemical examination and discussed them. All authors attended the discussion and have read and approved the final manuscript.
Acknowledgements
The authors thank Dr Tokuhiro Ishihara, Dr Toshiaki Kamei, and Dr Yosuke Nagahiro for their pathological analysis of this work.
References
- Siow SL, Chea CH, Hashimah AR, Ting SC (2011) Adult intussusception: 5-year experience in sarawak. Med J Malaysia 66:199-201. [Crossref]
[Google Scholar] [PubMed]
- Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919-932.
[Crossref] [Google Scholar] [PubMed]
- Lipton LR, Johnson V, Cummings C, Fisher S, Risby P, et al. (2004) Refining the Amsterdam criteria and bethesda guidelines: Testing algorithms for the prediction of mismatch repair mutation status in the familial cancer clinic. J Clin Oncol 22:4934-4943.
[Crossref] [Google Scholar] [PubMed]
- Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the international collaborative group on HNPCC. Gastroenterology 116:1453-1456.
[Crossref] [Google Scholar] [PubMed]
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, et al. (2004) Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96:261-268.
[Crossref] [Google Scholar] [PubMed]
- Peltomaki P (2005) Lynch syndrome genes. Fam Cancer 4:227-232.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, et al. (2013) Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology 144:926-932.
[Crossref] [Google Scholar] [PubMed]
- Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, Goossens M, Ouchene H, et al. (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in lynch syndrome-like tumors. Gastroenterology 146:643-466.
[Crossref] [Google Scholar] [PubMed]
- Kanesada K, Tsunedomi R, Hazama S, Ogihara H, Hamamoto Y, et al. (2023) Association between a single nucleotide polymorphism in the R3HCC1 gene and irinotecan toxicity. Cancer Med 12:4294-4305.
[Crossref] [Google Scholar] [PubMed]
- Zyla R, Graham T, Aronson M, Velsher L, Mrkonjic M, et al. (2021) MLH1 epimutation is a rare mechanism for lynch syndrome: A case report and review of the literature. Genes Chromosomes Cancer 60:635-639.
[Crossref] [Google Scholar] [PubMed]
- Omachi R, Kojima M, Miyake T, Ueki T, Iida H, et al. (2021) A case of intussusception due to juvenile colorectal cancer with suspected lynch syndrome. Gan To Kagaku Ryoho 48:2145-2147.
[Google Scholar] [PubMed]
- McLeod KG, Balasuriya HD, Hodder RJ (2021) Unusual case of intussuscepting right colon cancer in a 21-year-old with Lynch syndrome. ANZ J Surg 91:E413-E4.
[Crossref] [Google Scholar] [PubMed]
- Wright JP, Monson JRT, Albert MR (2023) Lynch syndrome diagnosed during pregnancy presenting as acute ileocolic intussusception in the third trimester. Am Surg 89:165-167.
[Crossref] [Google Scholar] [PubMed]
- Abildgaard AB, Nielsen SV, Bernstein I, Stein A, Lindorff-Larsen K, et al. (2023) Lynch syndrome, molecular mechanisms and variant classification. Br J Cancer 128:726-734.
[Crossref] [Google Scholar] [PubMed]
- Kang SY, Park CK, Chang DK, Kim JW, Son HJ, et al. (2015) Lynch-like syndrome: characterization and comparison with EPCAM deletion carriers. Int J Cancer 136:1568-1578.
[Crossref] [Google Scholar] [PubMed]
Citation: Ogata R, Hazama S, Fujii T, Hino K, Tsunedomi R, et al. (2024) A Case Report of Lynch-Like Syndrome Diagnosed due to Intussusception Treated with Laparoscopic Colectomy. Diagnos Pathol Open 9: 230. DOI: 10.4172/2476-2024.9.2.230
Copyright: © 2024 Ogata R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 654
- [From(publication date): 0-2024 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 466
- PDF downloads: 188