A Case Report Of Intrabiliary Metastasis from Colonic Malignancy in Lynch Syndrome a Rare Entity
Received: 01-Jun-2021 / Accepted Date: 09-Jun-2021 / Published Date: 15-Jun-2021 DOI: 10.4172/2167-7964.1000332
Abstract
Intrabiliary metastasis from carcinoma colon is a rare entity. The incidence of intrabiliary metastasis from carcinoma rectum is 12-15% (2). About 5% of intrabiliary metastasis is attributed to hereditary colorectal cancers; Lynch syndrome being the most common. We herein present a case of a 35-year-old male patient with intrabiliary metastasis. This patient is a known case of carcinoma rectum that underwent low anterior resection and hepatic metastatectomy at the age of 20. After 15 years, he presented with a metachronous malignancy in descending colon with abdominal lymph nodal metastasis. He also had rising bilirubin levels and was found to have intrabiliary infiltrates on MRI. The final diagnosis was confirmed on spy bite cholangioscopic biopsy. This discussion highlights rare occurrences of intrabiliary metastasis from carcinoma colon.
Introduction
The liver is the most common site of colo-rectal cancer, metastases occurring in approximately 30% of patients [1]. Liver metastases are rarely associated with bile duct dilatation. Biliary metastasis is a rare entity; about 5% of cases are attributed to hereditary colorectal cancers. Jhaveri et al. [2] showed that, in a cohort of 297 patients, the incidence of biliary dilatation with colorectal liver metastasis was 16.5%, whereas that with other tumours was only 3.0%.
Distant metastasis in colorectal cancers is due to haematogenous spread while local recurrence after primary resection is due to microscopic residual viable cells at the resection margin [1]. Optimal treatment of colorectal cancers is surgery with adjuvant or neoadjuvant chemo radiation.
Solitary liver metastasis developing years after resection may be misdiagnosed as a primary hepatic or biliary neoplasm. Radiological imaging characteristics of the liver metastases are variable depending on the histological differentiation of primary tumour. The lesions could be lobulated and well marginated or could have infiltrative margins. Delineation of the margins of lesion on multislice scanners is important for further management as underestimation of actual extent of intrabiliary metastasis results in incomplete tumour clearance [3].
There are improved long-term survival rates after complete hepatic resection along with advances in the field of chemo radiotherapy and newer treatment strategies [1].
Colorectal liver metastasis with biliary infiltration causes diagnostic challenges in imaging as well as management.
This article presents the spectrum of USG, MRI and MRCP findings of biliary involvement of metastatic colorectal liver metastasis.
Case Presentation
A 35-year-old male patient, a known case of carcinoma rectum presented with low backache, abdominal pain, fever and jaundice after 15 years. His complaints started in 2003 with bleeding PR and he was diagnosed to have rectal growth and hepatic metastasis in segment V and III. He underwent high anterior resection with hepatic metastatectomy. Preoperatively, there was a large bulky tumour involving upper and mid rectum infiltrating into the left pelvic wall and serosa of the urinary bladder. There were few fleshy lymph nodes at the root of inferior mesenteric artery. Two liver metastasis in segment V and III were found. Postoperative staging was Duke-D and adjuvant radiation was suggested along with chemotherapy with Inj. Oxaliplatin & 5-FU
He was asymptomatic and 15 years later, he presented again to another hospital with abdominal pain and vomiting. CT abdomen done outside showed obstructive growth in the middle 3rd position of descending colon. Colonoscopy was also done followed by limited colectomy with Hartmann’s procedure. Biopsy showed moderately differentiated adenocarcinoma with signet ring cells. Pathological staging was pT4aN2b.
He then came to our hospital for further treatment. Routine blood investigations showed elevated WBC counts (18.05 x 103 ul), elevated carcinoid embryonic antigen level (3151 ng/ml), elevated bilirubin (38.5 mg/dL) with altered LFT (AST 143 U/L, ALT 214 U/L, alkaline phosphatase 749 U/L).
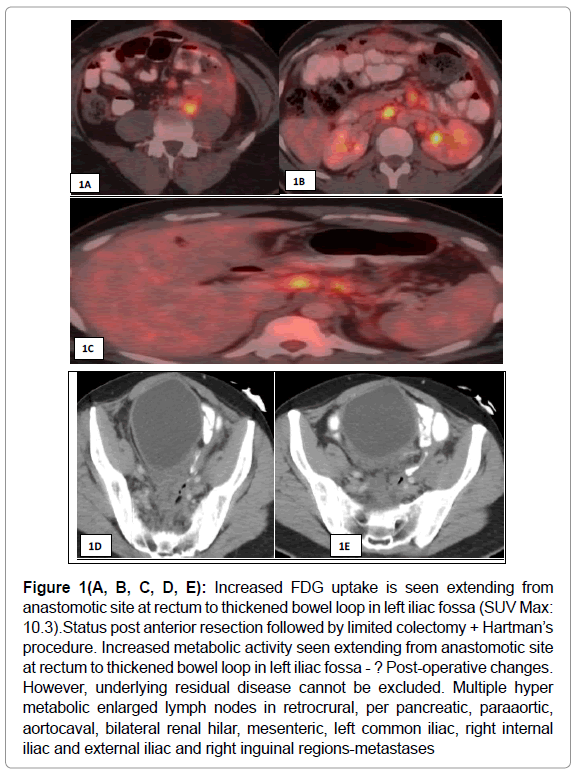
PET CT was done to assess the present status, which showed increased mild uptake at the postoperative site along with enlarged retroperitoneal and right inguinal metastasis. (Figure 1 (A, B, C, D, E) There was no evidence of metabolically active disease in the liver. There was a hyper metabolic prominent lymph node in the left hilar region.
Figure 1(A, B, C, D, E): Increased FDG uptake is seen extending from anastomotic site at rectum to thickened bowel loop in left iliac fossa (SUV Max: 10.3).Status post anterior resection followed by limited colectomy + Hartman’s procedure. Increased metabolic activity seen extending from anastomotic site at rectum to thickened bowel loop in left iliac fossa - ? Post-operative changes. However, underlying residual disease cannot be excluded. Multiple hyper metabolic enlarged lymph nodes in retrocrural, per pancreatic, paraaortic, aortocaval, bilateral renal hilar, mesenteric, left common iliac, right internal iliac and external iliac and right inguinal regions-metastases
Kras and Nras, BRAF mutation studies were done on the specimen which was negative. He was started on Folfox and Cetuximab. While on chemotherapy, he had disease progression and was put on immunotherapy with Pembrolizumab.
Later he developed hyperbilirubinemia and ultrasound followed by MRI and MRCP was done.
Imaging Features
Imaging Findings
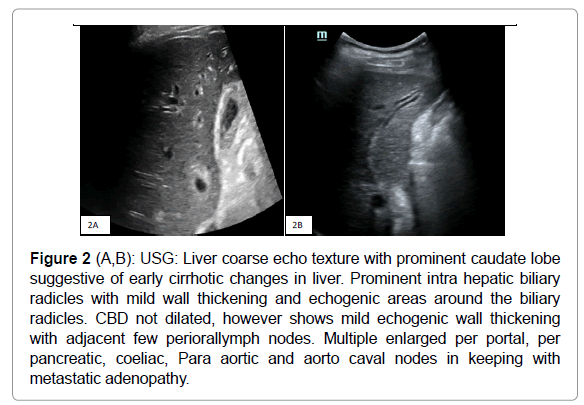
USG abdomen taken showed mild intrahepatic biliary radical dilatation with mild wall thickening and echogenicity along the biliary radicles, predominantly on the left side (Figure 2 A, B) Common bile duct was not dilated, however showed mild echogenic wall thickening. Multiple enlarged perioral, per pancreatic, coeliac, Para aortic and aorto caval nodes seen in keeping with metastatic adenopathy. Liver showed mild coarse echo texture with prominent caudate lobe, which was suggestive of early cirrhotic changes. There was mild left hydronephrosis, secondary to mass effect by the lymphadenopathy. By this time, patient had impaired RFT and hence, MRI was done to assess the cause of biliary dilatation.
Figure 2 (A,B): USG: Liver coarse echo texture with prominent caudate lobe suggestive of early cirrhotic changes in liver. Prominent intra hepatic biliary radicles with mild wall thickening and echogenic areas around the biliary radicles. CBD not dilated, however shows mild echogenic wall thickening with adjacent few periorallymph nodes. Multiple enlarged per portal, per pancreatic, coeliac, Para aortic and aorto caval nodes in keeping with metastatic adenopathy.
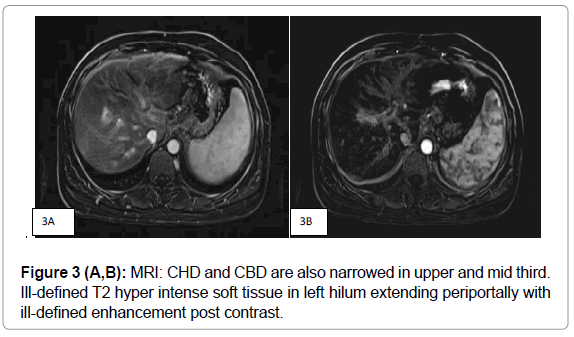
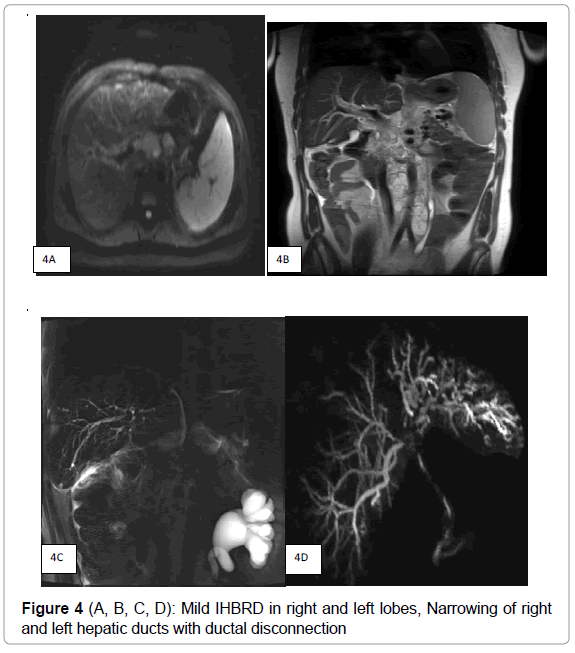
MRI showed minimal T2 hyper intense soft tissue along the hilum and left biliary radicles with ill-defined enhancement in post contrast study suggesting possibility of biliary deposits. There were no associated liver deposits. (Figure 3A, B) MRCP (Figure 4 A, B, C, D) showed mild intra hepatic biliary dilatation with diffuse narrowing predominantly of left hepatic duct with hilar stricture. CHD and CBD were also narrowed in upper and mid third due to multiple enlarged periportal, per pancreatic nodes. There was distant metastatic lymphadenopathy as evidenced by enlarged coeliac, Para aortic and aorto caval nodes. There was associated splenomegaly.
In view of hyperbilirubinemia, it was decided to go ahead with cholangioscopy followed by ERCP, stenting to evaluate of biliary tree and delineate the cause of obstruction. Cho angiogram showed irregular narrowing of the duct at the hilum with multiple strictures in the left duct with mild bilateral IHBRD. The differential considered was a periductal type of primary cholangiocarcinoma. Spyglass cholangioscopy showed erythema and friability in the hilum and left duct suspicious of tumour infiltration with prominent vessels. Spy bite biopsy was taken.
Histopathology
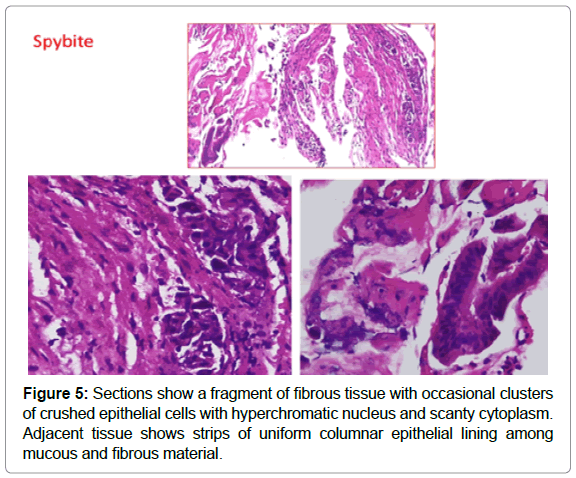
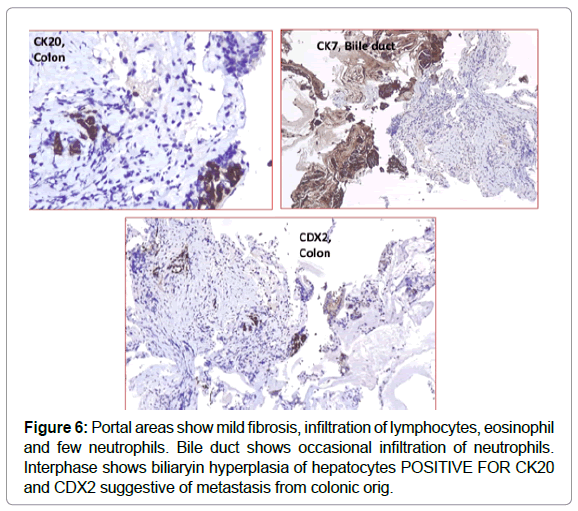
Histopathology with H&E followed by immunohistochemistry was done. The cells were positive for CK 20 and CD X2 and negative for CK7 confirming the diagnosis of intrabiliary metastasis, ruling out possibility of cholangiocarcinoma which is the closest differential [4] (Figures 5 & 6).
Immunohistochemistry (IHC) testing for Mismatch Repair Proteins (MMR) was done on the available specimen in our centre. There was loss of nuclear expression of MSH 2 and MSH 6. The tumour was DNA repair deficient with high frequency microsatellite instability with probability of Lynch syndrome; hence sequencing and/ or large deletion/ duplication testing of germ line mutation was suggested.
Confirmation genetic testing was done with ClinVar which showed mutation in the MSH-2 gene confirming diagnosis of Lynch syndrome.
Discussion
Intrabiliary growth of metastatic colorectal carcinoma is a rare entity, first reported in 1946 with symptomatic jaundice, biliary dilatation, and cirrhosis on autopsy. Study from MD Anderson Cancer Centre revealed that the intrabiliary growth of hepatic metastasis are predominantly from colorectal carcinoma (93%), among which recto sigmoid colon is the most common site of the primary tumour [5].
The main conclusion of several reports [6-10] is that if there is biliary duct dilatation and intrabiliary growth, with a history of colorectal cancer, liver metastases must be considered as the most likely diagnosis even when histological and radiological features indicate an intrahepatic cholangiocarcinoma.
Mechanism of spread is shown in Figure 7.
Figure 7: Mechanism of intrahepatic bile ducts metastasis from colorectal cancer. Cancer cells may spread from the primary colorectal cancer via the portal circulation and implant in the bile duct by passing through the per biliary capillary plexus [11].
Two patterns of intrabiliary metastasis are described: a) colonization of the bile duct and b) tumor floating and blocking the ductal lumen, with each pattern comprising 81% of the cases [12] (Figure 7). Pathology reports favour colonizing pattern in our case.
Studies performed by Povoski et al. and Estella et al. indicates that there are no significant differences in survival rate and differentiation between metastatic deposits in major and minor bile ducts [9] in colorectal cancer.
Hereditary colorectal cancer accounts around 2 to 5% of all cancer cases in the Western world although the incidence is relatively low in the Indian subcontinent. Amongst all, Lynch syndrome is the leading cause. The age of onset of malignancy is earlier and the lesions are more proximally located in cases of Lynch syndrome.
Hereditary colorectal cancer is suspected when [13]
Colon cancer diagnosed < 50 years of age
Multiple colonic malignancies present, whether synchronous or metachronous
Multiple primary cancers diagnosed either colonic or extra colonic
Over a lifetime >10 adenomas present or >2 histologically characterized hamartomatous polyps.
Colon cancer in > 1 generation of the individual’s family
Clustering of extra colonic cancers in family members.
Amsterdam II criteria and revised Bethesda criteria are used to confirm hereditary cancer syndrome in at risk individuals along with gene testing. National Cancer Care Network (NCCN) guidelines recommend colonoscopy between age group 20 to 25 years or 2 to 5 years prior to the earliest colon cancer detected with repeat colonoscopy every 1-2 years.
In our case, detailed family history revealed history of carcinoma stomach in the grandfather and carcinoma colon in the father. Along with very young age and history of multiple carcinomas in the family, possibility of Lynch syndrome was confirmed with Amsterdam II criteria and genetic studies.
Our case is unique with the presence of intrabiliary metastasis from colorectal cancer. A review of literature shows multiple case reports of intrabiliary metastasis from colorectal cancer but has not been described in Lynch Syndrome 1. The longest duration between initial resection and metastasis as per literature, is 12 years [14] in our case, the time duration between initial resection and presentation of the intrabiliary growth was 15 years.
Conclusion
We have presented a rare case of intrabiliary metastatic growth liver from colon cancer that occurred 15 years after radical colectomy. Though, liver metastasis can cause secondary biliary involvement, in our case the metastatic deposit was involving the biliary tree without hepatic involvement which was proven on cholangioscopic biopsy. Immunohistochemical staining for CK7, CK20, and CD X2 confirmed the diagnosis. Delineation of the extent of metastasis is important in preoperative surgical planning and further treatment as incomplete resection may result in recurrence.
References
- Coppola S, Zucchini N, Romano F, Bovo G, Gilardoni E, et al. (2014) colorectal liver metastasis with intrabiliary growth: case report and review of the literature. Int J Surg Pathol 22(3):272–279.
- Intrabiliary Growth of Colorectal Liver Metastasis: (2013) Spectrum of Imaging Findings and Implications for Surgical Management. AJR, 201:W582–W589.
- Jhaveri KS, Halankar J, Aguirre D (2009) Intrahepatic bile duct dilatation due to liver metastases from colorectal carcinoma. AJR193:752–756.
- Hirotaka Tokai, Yujo Kawashita, Susumu Eguchi, Yukio Kamohara, Mitsuhisa Takatsuki, et al. (2006)World J Gastroenterol 12(30): 4918–4921
- Maharaj R, Shukla P, Sakpal S, Naraynsingh V, Dan D, et.al (2014) The impact of hereditary colorectal cancer on the Indian population. Indian J Cancer 51:538-541
- Ballester V, Cruz-Correa M. (2018) How and When to Consider Genetic Testing for Colon Cancer? Gastroenterology.155:955-9.
- Sasaki S, Nomura Y, Fukutomi S, Shirahama N, Kusano H, et al. (2019)intrabiliary growth type of metastasis from colon cancer, 12 years after curative colectomy: a case report. BMC Surg 19:8
- Sasaki, Yoriko Nomura, Shogo Fukutomi, Nobuhisa Shirahama, Hironori Kusano2 et al. (2019) intrabiliary growth type of metastasis from colon cancer, 12 years after curative colectomy: a case report Shin BMC Surgery 19:8.
- Estrella JS, Othman ML (2013) Taggart, Intrabiliary growth of liver metastases clinic pathologic features, prevalence, and outcome,†The American Journal of Surgical Pathology 10;37: 1571–1579
- Ghittoni G, Caturelli E, Viera FT (2010) Intrabile duct metastasis from colonic adenocarcinoma without liver parenchyma involvement: contrast enhanced ultrasonography detection. Abdom Imaging 35:346-348. 11.
- Riopel MA, Klimstra DS, Godellas CV, Blumgart LH, Westra WH (1997) Intrabiliary growth of metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol 21:1030-1036.
- Fraguela Mariña JA, Fernández Blanco C, Alonso Fernández L, Taboada Filgueira L, Robles Veiga O, et.al (2007) Liver metastases from colonic adenocarcinoma simulating cholangiocarcinoma Gastroenterol Hepatol 30:454-456.
- Uehara K, Hasegawa H, Ogiso S (2004) Intrabiliary polypoid growth of liver metastasis from colonic adenocarcinoma with minimal invasion of the liver parenchyma. J Gastroenterol 39:72-75.
- Seshadri RA, Majhi U (2009) Endobiliary metastasis from rectal cancer mimicking intrahepatic cholangiocarcinoma: a case report and review of literature. J Gastrointest Cancer 40:123-127.
Citation: Shabir M, Pratap T (2021) A Case Report Of Intrabiliary Metastasis from Colonic Malignancy in Lynch Syndrome a Rare Entity . OMICS J Radiol 10: 332. DOI: 10.4172/2167-7964.1000332
Copyright: © 2021 Shabir M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2322
- [From(publication date): 0-2021 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 1597
- PDF downloads: 725