A Case of Vas Deferens Lesion with Features of Vasitis Nodosa and Mesothelial Proliferationc
Received: 09-Nov-2020 / Accepted Date: 23-Nov-2020 / Published Date: 30-Nov-2020
Abstract
Background: Vasitis nodosa is a nodular lesion within the vas deferens that is characterized by a benign glandular proliferation of the vas deferens epithelium mixed with sperm and chronic inflammation. It is considered a reactivecondition caused by increased intraluminal pressure. Problems in diagnosis can arise when atypical morphologic features are present.
Case presentation: A 25-year old African American male status post vasectomy was diagnosed with obstructive azoospermia and elected to undergo a vasovasostomy. The morphology of the resected specimen was consistent with vasitis nodosa, but the proliferative epithelial cells also demonstrated the phenotype of mesothelium by immunohistochemistry.
Conclusion: This is the first reported case of vas deferens lesion with both features of vasitis nodosa and mesothelial proliferation, and it may give insight into the cellular origin of vasitis nodosa.
Keywords: Vasitis nodosa; Biphenotypic; Mesothelium
Background
Vasitis nodosa is a terminology first used by Benjamin et al to describe a lesion in the vas deferens that reflects its nodular macroscopic appearance and potential inflammatory nature. The mechanism is believed to be due to increased intraluminal pressure as a result of obstruction and subsequent cascade of spermatic leakage and tubular proliferation [1]. Vasitis nodosa has been most often been seen in patients status post vasectomy and associated with spontaneous recanalization [2]. Patients are mainly asymptomatic and require no clinical intervention [1]. Although rare painful lesions have been reported [2]. Vasitis nodosa may occasionally be misinterpreted as a malignant lesion or inguinal hernia clinically, and it is resected when it presents as a mass or painful lesion [3-6].
The histology of vasitis nodosa is characterized by mural nodules composed of extravasated spermatozoa accompanied by the formation of epithelial-lined spaces [7]. The lesion is usually associated with fibrosis, chronic inflammation, and sperm granulomas [8]. Although it is a benign condition, vasitis nodosa demonstrates several features of malignancy [1,9,10]. Overdiagnosis of malignancy may occur if one is not familiar with these variations. However, metastatic tumors can colonize vasitis nodosa, and some tumors can even mimic vasitis nodosa [11-13]. Diagnosis of these tumors can be challenging especially when overwhelming tubular proliferation and inflammation are present.
It believed that the immunoprofile of the tubules in vasitis nodosa reflects the phenotype of vas deferens epithelium except for the increased expression of AMACR (P504S) [11]. Our case of vasitis nodosa, however, also expresses mesothelial markers. This knowledge expands the known immunoprofile of vasitis nodosa and suggests least some of the vasitis nodosa may represent mesothelial proliferation.
Case presentation
Clinical history
The patient is a 25-year old African American male married with two kids, who underwent a vasectomy in March of 2017. Subsequently, he and his wife desired to have children. He was counseled on options including vasovasostomy versus microsurgical epididymal sperm aspiration/testicular sperm aspiration with in vitro fertilization. The patient and his wife elected for a vasovasostomy in September of 2019. Intraoperative findings included bilateral segments of atretic vas deferens, spermatic granuloma on the left and clear fluid from the testicular end of the vas which demonstrates lack of sperm under microscope. Semen analysis was scheduled for approximately 6 weeks to 2 months post operation.
Histopathology
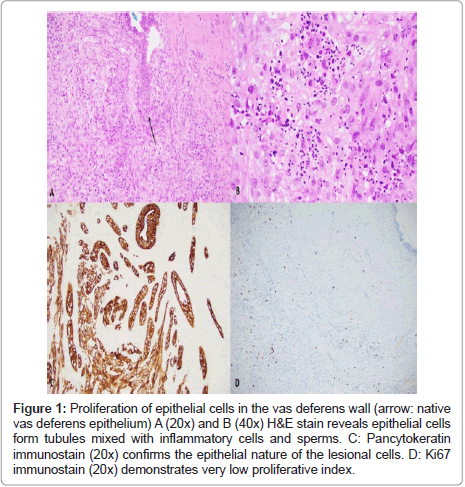
Histologically, the stenosis of the vas deferens was due to multiple unencapsulated nodules with seemingly infiltrative borders. The nodules were centered on the lumen and composed of florid proliferation of anastomosing tubules mixed with sperm and chronic inflammation involving the muscle and adventitia. These tubules were composed of syncytial and streaming epithelial cells with clear to eosinophilic cytoplasm, monotonous nuclei, and conspicuous nucleoli with fine chromatin, and a smooth nuclear membrane. The tubule cells stained positive for pan cytokeratin. Occasional hyaline globules and rare mitoses were present. The tubule cells demonstrated a very low proliferative index (Figure 1) the inflammatory cells were predominantly histiocytic which were highlighted by the CD68 (KP1) antibody. Several spermatic granulomas were also identified. No lymph vascular or perineural invasion was identified. The morphology was consistent with vasitis nodosa. The opposite vas deferens showed similar but less florid tubular proliferation.
Figure 1: Proliferation of epithelial cells in the vas deferens wall (arrow: native vas deferens epithelium) A (20x) and B (40x) H&E stain reveals epithelial cells form tubules mixed with inflammatory cells and sperms. C: Pancytokeratin immunostain (20x) confirms the epithelial nature of the lesional cells. D: Ki67 immunostain (20x) demonstrates very low proliferative index.
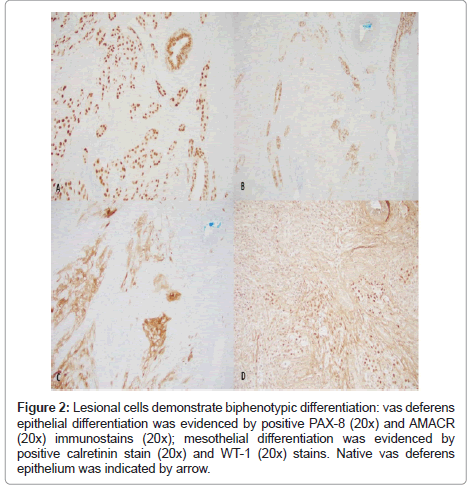
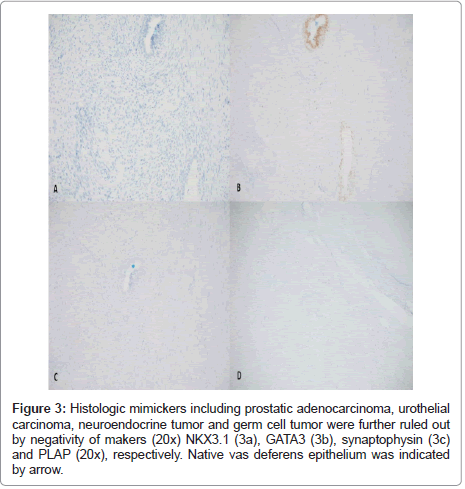
Immunohistochemically stains were used to delineate the lineage of differentiation (Figure 2).The lesional tubule cells demonstrated positive reactivity to PAX-8 antibody. AMACR (P504S) was overexpressed in comparison to vas deferens epithelium, which was weakly positive. WT-1 and calretinin were initially performed to rule out a mesothelial lesion such as adenomatoid tumor and mesothelioma which are common lesions in this anatomical location. Both markers were positive in the lesional cells. Several other organ-specific markers including NKX3.1, GATA3, PLAP and synaptophysin were also performed to exclude metastatic prostatic, urothelial, germ cell, and neuroendocrine tumors and found to be negative (Figure 3).
Figure 2: Lesional cells demonstrate biphenotypic differentiation: vas deferens epithelial differentiation was evidenced by positive PAX-8 (20x) and AMACR (20x) immunostains (20x); mesothelial differentiation was evidenced by positive calretinin stain (20x) and WT-1 (20x) stains. Native vas deferens epithelium was indicated by arrow.
Figure 3: Histologic mimickers including prostatic adenocarcinoma, urothelial carcinoma, neuroendocrine tumor and germ cell tumor were further ruled out by negativity of makers (20x) NKX3.1 (3a), GATA3 (3b), synaptophysin (3c) and PLAP (20x), respectively. Native vas deferens epithelium was indicated by arrow.
Results and Discussion
Various tumor and tumor-like lesions can occur in near the testes and contain epithelial components with adenomatoid tumor being the most common[14]. Unlike vasitis nodosa, the epithelium of adenomatoid tumor is flattened, and the stroma is fibrotic[14]. Mesothelial proliferation can also occur in tunica vaginalis and can occasionally be tubular [15]. However, mesothelial lesions are typically centered on the serosa (tunica vaginalis) instead of the lumen of the vas deferens, as was seen with our cases [15]. It would be rare for mesothelial lesions to spare the surrounding connective tissue since the vas deferens is not directly enveloped by serosa. Instead, the serosa (tunica vaginalis) wraps around the entire spermatic cord including the connective tissue. Also, mesothelial proliferation consists of a fibrotic stroma which is absent in our case. Therefore, morphologically our case is most consistent with a vasitis nodosa.
Similar to the lining epithelium of the vas deferens, the epithelial cells of vasitis nodosa are positive for cytokeratin 7 and 19, PAX8, CD10 and vimentin with patchy expression of GATA3 [8,11]. High molecular weight keratin 34 beta E12 is present in the basal lining cells of the vas deferens and vasitis nodosa [8]. P63 is positive in basal cells of vas deferens lining epithelium but show patchy expression in vasitis nodosa [11]. In contrast to the lining cells, some vasitis nodosa cells are positive for CA125 and AMACR [11]. Other prostate markers such as PSA, prostein and NKX3.1 are consistently negative [11]. However, none of these markers are specific for epithelium of vas deferens, and they can be positive in at least some mesothelial lesions [16,17]. Our case demonstrates definitive expression of mesothelial markers in a morphologically typical vasitis nodosa. This brings up the suggestion that either our vasitis nodosa case is a mesothelial lesion in nature or shows biphenotypic differentiation with phenotypes of vas deferens epithelium and mesothelium.
Vasitis nodosa and its counterpart, epididymis epididymitis nodosa [18] were named for their resemblance to salpingitis isthmica nodosa [7]. Vasitis nodosa is the most common asymptomatic postoperative complication of a vasectomy and is identified in 50%-66% of the patients with the obstruction of the proximal end and associated inflammation [1,19]. Most cases of vasitis nodosa occurred within 2 months to 19 year post operation [20]. It was thought to be a result of a breach in the lining of the epithelium due to increased intravasal pressure and subsequent epithelial regeneration [21]. This is could be attributed to an attempt at re-establishing the communication in an obstructed lumen and restoring the reproductive capacity of the individual [22]. Recanalization has been reported in the presence of vasitis nodosa [23]. Similarly, the spermatic granulomas represent the body’s attempt to restore fertility by allowing sperm to leak into the surrounding tissue [24]. This hypothesis was indirectly supported by the observation that a better surgical sealing of the cut ends lead to a decreased incidence of vasitis nodosa and spermatic granulomas. Vasitis nodosa has also been identified in patients who are status post herniorrhaphy or have chronic inflammation or a bladder diverticulum or experience trauma [1,3,25]. Vasitis nodosa can rarely arise in patients with none of these risk factors, and these lesions usually lack inflammation [3,20]. Presence of spermatic granulomas was thought to be associated with a higher chance of fertility and impregnation [26]. However, this was not successfully reproduced in a later study [1]. The persistent infertility after vasectomy reversal might be attributed to the development of antisperm antibodies which have been detected in 50%-70% patients after vasectomy [1].
The correct diagnosis of vasitis nodosa relies on the recognition of a benign process with seemingly malignant morphologic features. The florid proliferative activity of the epithelium can be misleading and easily misinterpreted as a malignant process. Atypical cytologic features such as vesicular nuclei, prominent nucleoli and mitotic figures can occasionally be present [19]. The lesion can extend to involve the muscle layer and adventitia [1,27,28]. Benign perineural and intraneural invasions have also been reported [4,9,19,29]. “Benign neural invasion” has been identified in 16% of vasitis nodosa cases in one study in which one or two nerves were invaded with one to eight glands [10]. This was further complicated by nerve hyperplasia and neuromas. This phenomenon might be due to nerve growth factor expression which has been demonstrated in the epithelium of vasitis nodosa immunohistochemically [30]. The epithelium in vasitis nodosa can also invade small veins or arteries which are usually accompanied by elastosis [31]. It has been postulated that proliferating ductules in vasitis nodosa invade the blood vessels after they have become obliterated by regressive and reparative processes.
Tumors can also mimic or colonize vasitis nodosa. Tumors especially of adjacent organs, such as prostatic adenocarcinoma and urothelial carcinoma with glandular differentiation can mimic the epithelial proliferation of vasitis nodosa [11]. Presence of seminoma cells in the stroma or pagetoid spread inside tubules with concurrent testicular seminoma has been reported [12]. Since no lymphatic, perineural, epididymis, or proximal vas deferens involvement has been demonstrated, it is hypothesized that the tumor cells spread by shedding and subsequent arresting [12]. A similar case of involvement of vasitis nodosa by germ cell tumors demonstrated unclassified germ cell neoplasia with pagetoid spread involving varsities nodosa. The concurrent germ cell tumor was in the ipsilateral testis and contained no seminoma component [13,20].
Conclusion
In conclusion, we report for the first time a case of morphologically typical vasitis nodosa expressing mesothelial markers. Electron microscopy may be useful in confirming or eliminating the mesothelial nature of the proliferating cells in these lesions.
Declarations
Acknowledgements
Not applicable.
Authors’ contributions
B.R. diagnosed the surgical samples pathologically and wrote the manuscript.
W.L. diagnosed the surgical samples pathologically and revised the manuscript.
All authors have read and approved the final manuscript.
Availability of data and materials
The surgical materials and the datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent for publication of clinical history was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Ethics approval and consent to participate
Not applicable.
Funding
The authors have no funding to disclose.
References
- Hirschowitz LY, Rode J, Guillebaud J, Bounds W, Moss EI (1998)Â Vasitis nodosa and associated clinical findings. J Clin Pathol 41: 419-423.
- Kiser GC, Fuchs EF,Kessler S (1986) The significance of vasitis nodosa. J Urol 136: 42-44.
- Warner JJ, Kirchner FK, Wong SW, Dao AH (1983)Â Vasitis nodosa presenting as a mass of the spermatic cord. J Urol 129: 380-381.
- Goldman RL, Azzopardi JG (1982) Benign neural invasion in vasitis nodosa. Histopathology 6: 309-315
- Ash L, Hatem S, Ramirez GA, Veniero J (2019) Vasitis: A clinical confusion diagnosis with inguinal hernia. Int Braz J Urol 45: 637-638.
- Eddy K, Piercy GB, Eddy R (2011) Vasitis: clinical and ultrasound confusion with inguinal hernia clarified by computed tomography. Can Urol Assoc J 5: E74-E76.
- Sakaki M, Hirokawa M, Horiguchi H, Wakatsuki S, Sano T (2000) Vasitis nodosa: Immunohistochemical findings-case report. APMIS 108: 283-286.
- Zimmerman KG, Johnson PC, Paplanus SH (1983) Nerve invasion by benign proliferating ductules in vasitis nodosa. Cancer 51: 2066-2069.
- Balogh K, Travis WD (1984) The frequency of perineurial ductules in vasitis nodosa. Am J Clin Pathol 82: 710-713.
- Kezlarian BE, Cheng L, Gupta NS, Williamson SR (2018) Vasitis nodosa and related lesions: A modern immunohistochemical staining profile with special emphasis on novel diagnostic dilemmas. Hum Pathol 73: 164-170.
- Heaton JM, MacLennan KA (1986) Vasitis nodosa-a site of arrest of malignant germ cells. Histopathol 10: 981-989.
- Khamu TT, Strutton GM, Kiosoglous A (2010) Â Pagetoid involvement of vasitis nodosa by intratubular germ cell neoplasia unclassified. Pathol 40: 690-692.
- Khandeparkar SG, Pinto RG (2015) Histopathological Spectrum of Tumor and Tumor-like Lesions of the Paratestis in a Tertiary Care Hospital. Oman Med J 30: 461-468.
- Lee S, Illei PB, Han JS, Epstein JI (2014) Florid mesothelial hyperplasia of the tunica vaginalis mimicking malignant mesothelioma: a clinicopathologic study of 12 cases. Am J Surg Pathol 38: 54-59.
- Chapel DB, Husain AN, Krausz T, McGregor SM (2017) PAX8 Expression in a Subset of Malignant Peritoneal Mesotheliomas and Benign Mesothelium has Diagnostic Implications in the Differential Diagnosis of Ovarian Serous Carcinoma. Am J Surg Pathol 41: 1675-1682.
- Duyar SS, Yilmaz A, Demirağ F, Erdoğan Y, Yazici Ü, et al. (2015) The expression and clinical effects of alpha-methylacyl-CoA racemase (AMACR/ P504S) as an immunohistochemical marker in malign pleural mesothelioma. Turk J Med Sci 45: 607-614.
- Schned AR, Selikowitz SM (1986) Epididymitis nodosa. An epididymal lesion analogous to vasitis nodosa. Arch Pathol Lab Med 110: 61-64.
- 19.Taxy JB, Marshall FF, Erlichman RJ (1981) Vasectomy: subclinical pathologic changes. Am J Surg Pathol 5: 767-772.
- Taxy JB (1978) Vasitis nodosa. Two cases. Arch Pathol Lab Med 102: 643-647.
- Balogh K, Argenyi ZB (1985) Vasitis nodosa and spermatic granuloma of the skin: an histologic study of a rare complication of vasectomy. J Cutan Pathol 12: 528-533.
- Deshpande RB, Deshpande JJ, Mali BN, Kinare SG (1985) Vasitis nodosa (a report of  7 cases). J Postgrad Med 31: 105-108.
- Hirschowitz LY, Rode J, Guillebaud J, Bounds W, Moss EI (1997) [Vasitis nodosa]. Arch Esp Urol 50: 534-536.
- Schmidt SS, Minckler TM (1992) The vas after vasectomy: Comparison of cauterization methods. Urology 40: 468-70.
- Ralph DJ, Lynch MJ, PryorJP (1993) Vasitis nodosa due to torture. Br J Urol 72: 515-516.
- Silber SJ (1977) Sperm granuloma and reversibility of vasectomy. Lancet 2: 588-589.
- Onishi N, Honjoh M, Takeyama M, Sakaguchi H (1992) Vasitis nodosa suspected of the spermatic cord tumor: A case report. Hinyokika Kiyo 38: 595-597.
- Onishi N, Honjoh M, Takeyama M, Sakaguchi H(1991) [Vasitis nodosa: Report of two cases]. Nihon Hinyokika Gakkai Zasshi. Japanese J Urol 82: 645-648.
- Kovi J, Agbata A (1974) Letter: Benign neural invasion in vasitis nodosa. JAMA 228: 1519.
- DeSchryver-Kecskemeti K, Balogh K, Neet KE (1987) Â Nerve growth factor and the concept of neural-epithelial interactions. Immunohistochemical observations in two cases of vasitis nodosa and six cases of prostatic adenocarcinoma. Arch Pathol Lab Med 111: 833-835.
- Balogh K,Travis WD (1985) Benign vascular invasion in vasitis nodosa. Am J Clin Pathol 83: 426-430.
Citation: Luo W, Raju B (2020) A Case of Vas Deferens Lesion with Features of Vasitis Nodosa and Mesothelial Proliferation. J Clin Exp Pathol 10: 386.
Copyright: © 2020 Luo W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3061
- [From(publication date): 0-2020 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 2334
- PDF downloads: 727