Research Article Open Access
A Case of Pancreaticopleural Fistula Successfully Treated with a Pancreatic Duct Stent
Sébastien Desnoyers*
Gastroenterology Unit, Medicine Department, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Québec, Canada
- Corresponding Author:
- Sebastien Desnoyers MD
Gastroenterology Unit, Medicine Department
Centre Hospitalier Universitaire de Sherbrooke
Sherbrooke, Québec, Canada
Tel: 1-819-346-1110
Fax: 1-819-822-6706
E-mail: sebastien.desnoyers@usherbrooke.ca
Received Date: January 25, 2016 Accepted Date: March 2, 2016 Published Date: March 09, 2016
Citation: Desnoyers S (2016) A Case of Pancreaticopleural Fistula Successfully Treated with a Pancreatic Duct Stent. J Gastrointest Dig Syst 6:396. doi:10.4172/2161-069X.1000396
Copyright: © 2016 Desnoyers S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Pancreaticopleural fistula (PPF) is a rare complication of chronic pancreatitis. We present the case of a 34 yearsold male who presented with a massive left side pleural effusion. The MRCP showed the PPF and the patient was rapidly treated with a pancreatic duct stent. There was no recurrence of pleural effusion after twenty-two months of follow-up. This case highlights the prompt recognition of PPF by medical team and the right identification of a fistula amenable to an endoscopic treatment.
Keywords
Pancreaticopleural fistula; Pancreatitis; Pleural effusion
Introduction
Pancreaticopleural fistula (PPF) is a rare complication of pancreatitis, occurring in 0.4% of patients with pancreatitis and in 4.5% of patients with pancreatic pseudocyst [1,2]. Most of the PPF form by leak of pancreatic secretion from a pancreatic pseudocyst. Rare cases occur directly thru the leaking of a pancreatic duct [1,3] We present a case of PPF that was successfully treated with a pancreatic duct stent.
Case Description
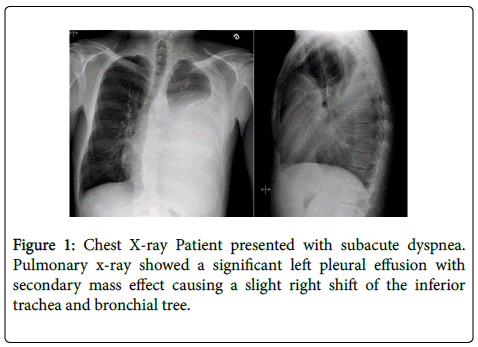
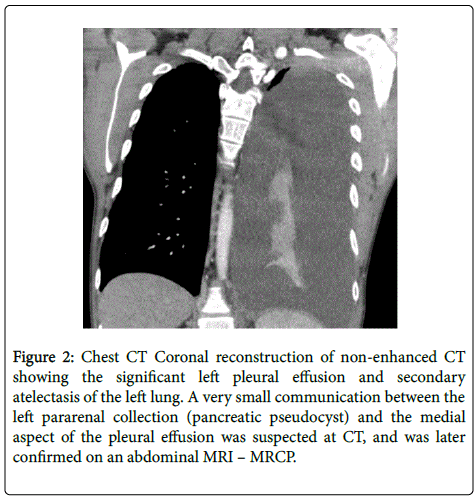
A 34 year-old male with a past history of alcohol-induced chronic pancreatitis was admitted to our hospital with a three weeks course of dyspnea. Physical examination of the patients was notable for diminished air passage and dullness on percussion of left chest wall. Chest X-ray revealed massive pleural effusion on the left side that was confirmed by contrastenhanced CT of the chest (Figures 1 and 2).
Figure 2: Chest CT Coronal reconstruction of non-enhanced CT showing the significant left pleural effusion and secondary atelectasis of the left lung. A very small communication between the left pararenal collection (pancreatic pseudocyst) and the medial aspect of the pleural effusion was suspected at CT, and was later confirmed on an abdominal MRI – MRCP.
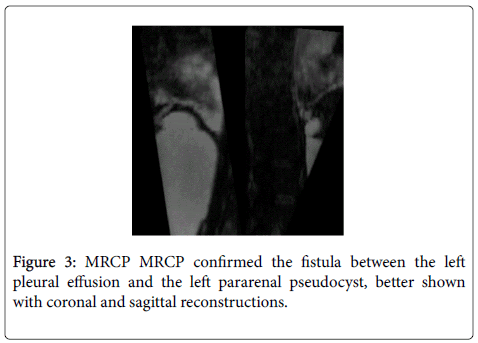
Pleural tap recovered 1.5 L of black-colored fluid with amylase levels above 30 000 IU/L, which was much higher than the serum amylase level (1082 IU/L). MRCP showed a retroperitoneal pseudocyst (3.1 X 9.0 X 14.7 cm) near the head of the pancreas communicating with the left pleural space through a 5 mm fistula in the left diaphragm (Figure 3). No connection between the pseudocyst and the duct of Wirsung was seen on the MRCP. This exam also revealed a stenosis of Wirsung duct in the pancreatic body. A first ERCP attempt failed to cannulate the Wirsung duct. Initial treatment consisted of octreotide and enteral nutrition.
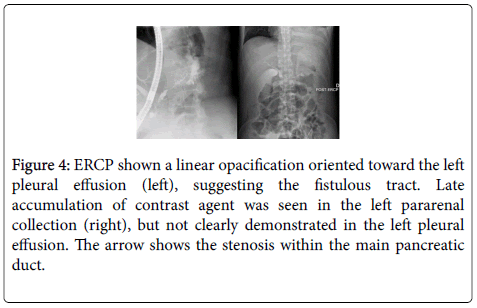
During the second ERCP attempt, 20 days after the patient’s admission, a stenosis in the middle part of the Wirsung and a dilation of the distal part of the duct was discovered with contrast extravasion communicating with the pseudocyst (Figure 4). At this time, a pancreatic duct stent (7-french, 12 cm) was inserted to cover the site of duct disruption and bridge the Wirsung stenosis. On day 34, MRCP showed near-complete resolution of the pseudocyst. The pancreatic duct stent was changed 4 times after the first installation and patient had a stent for a total of 15 months. Follow-up of the patient’s condition twenty-two months after the diagnosis of PPF showed no return of pleural effusion.
Figure 4: ERCP shown a linear opacification oriented toward the left pleural effusion (left), suggesting the fistulous tract. Late accumulation of contrast agent was seen in the left pararenal collection (right), but not clearly demonstrated in the left pleural effusion. The arrow shows the stenosis within the main pancreatic duct.
Discussion
Pancreaticopleural fistula is a rare condition particularly affecting middle-aged men with alcohol-induced chronic pancreatitis with pancreatic pseudocyst [1-3]. More than 60% of patient will be dyspneic at the clinical presentation [1]. There is often a delay between clinical presentation and diagnosis because of the severity of respiratory complaint presented by patient. Therefore, clinician must be suspicious for PPF with patient known for chronic pancreatitis disease and presenting with a new pleural effusion [2,3]. The most important diagnostic test for PPF is an exudative pleural effusion with a high amylase count. No threshold of amylase level is described in the literature to confirm a diagnosis of PPF but levels above 50 000 IU/L are only described in that condition. Clinicians should remember that others conditions can present with a high amylase count in a pleural tap: acute pancreatitis, pneumonia, cancer, oesophagus perforation and pulmonary tuberculosis [1-3]. The sensitivity of CT scans to detect pancreaticopleural fistula is low in comparison to MRCP and ERCP that have sensitivities around 80% [1]. MRCP seems to be the exam of choice for diagnosing PPF. MRCP is a non-invasive tool that provides image of whole pancreas and possible pseudocyst. Compare to ERCP, MRCP allow physician to see the Wirsung duct above a stricture site or lithiasis [2,4]. ERCP is a user-dependent technique with possible complications like pancreatitis, infection and haemorrhage risks [2,5]. Management options for PPF consist of conservative treatment, including endoscopic procedures, or surgery. Traditionally, a medical treatment based on fasting state and somatostatin analogue was initiated to reduce the fistula fluid output [1]. This treatment was first attempted for 2-3 weeks [1]. Previous case series suggest a success rate between 30 and 65% for medical treatment alone [1,2,5-7]. Observational data suggest that medical treatment alone is associated with longer hospitalisation time and more complications because of the low rate of spontaneous resolution of the fistula [1,7,8]. Actually, the key point for treatment of PPF is based on the pancreas duct anatomy of the patient, especially on the identification of duct stricture and site of duct disruption [2,5,9]. The goal of pancreatic duct stenting with ERCP is to produce a low resistance path to alleviate the fistula and to mechanically close the fistula track with the stent [2,5]. Characteristics of fistula that are amenable to endoscopic treatment include identification of a duct disruption in the head or body of the pancreas and a stricture downstream from the duct disruption [2,5,10]. Case series results are inconsistent with regard to the success rate of pancreaticoduodenal stenting [5]. The duration that a pancreatic duct stent therapy should be performed is unknown. Some authors suggest performing an ERCP every 6 weeks to assess resolution of the fistula [7,10]. Failure or inability to install a duct stent or complication of pleural effusion by an infection are ground for consultation with a surgeon [1,2,5].
Conclusion
This clinical case highlights a rare clinical condition that was promptly recognized by the medical team. The key point in managing this condition is to recognize which patient could benefit of an endoscopic treatment with a pancreatic duct stent. Despite the growing number of cases of PPF treated in endoscopy, multiple questions about the technique stay unanswered. One of these questions is what should be the duration of stenting to alleviate the fistula [7-10]. The burden of PPF cases report like this one could give clues to these questions.
Acknowledgement
We would like to thank physicians of our hospital having contributed to this article: Jean-Daniel Baillargeon, Annie Beaudoin, Étienne Couture, Caroline Lacroix, Yannick Poulin, Nathalie Voyer.
References
- Ali T, Srinivasan N, Le V, Chimpiri AR, Tierney WM (2009) Pancreaticopleural fistula. Pancreas 38: e26-31.
- Tay CM, Chang SK (2013) Diagnosis and management of pancreaticopleural fistula. Singapore Med J 4: 190-194.
- Machado NO (2012) Pancreaticopleural fistula: revisited. Diagn Ther Endosc 2012: 815476.
- Vyas S, Gogoi D, Sinha SK, Singh P, Yadav TD, et al. (2009) Pancreaticopleural fistula: an unusual complication of pancreatitis diagnosed with magnetic resonance cholangiopancreatography. JOP 10: 671-673.
- Wronski M, Slodkowski M, Cebulski W, Moronczyk D, Krasnodebski IW (2011) Optimizing management of pancreaticopleural fistulas. World J Gastroenterol 17: 4696-4703.
- Koshitani T, Uehara Y, Yasu T, Yamashita Y, Kirishima T, et al. (2006) Endoscopic management of pancreaticopleural fistulas: a report of three patients. Endoscopy 38: 749-751.
- Dhebri AR, Ferran N (2005) Nonsurgical management of pancreaticopleural fistula. JOP 6: 152-161.
- King JC, Reber HA, Shiraga S, Hines OJ (2010) Pancreatic-pleural fistula is best managed by early operative intervention. Surgery 147: 154-159.
- Roberts KJ, Sheridan M, Morris-Stiff G, Smith AM (2012) Pancreaticopleural fistula: etiology, treatment and long-term follow-up. Hepatobiliary Pancreat Dis Int 11: 215-219.
- Aswani Y, Hira P (2015) Pancreaticopleural fistula: a review. JOP 16: 90-94.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 12739
- [From(publication date):
April-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11811
- PDF downloads : 928