Case Report Open Access
A Case of Morganella morganii Meningoencephatitis
| Tomoyuki Nakazawa1* Kaoru Obinata1 Yuko Nagata1 Kana Ebara1 Kyoko Suzuki1 Toshiaki Shimizu2 | ||
| 1Department of Pediatrics, Juntendo University Urayasu Hospital, Chiba, Japan | ||
| 2Department of Pediatrics, Juntendo University Faculty of Medicine, Tokyo, Japan | ||
| Corresponding Author : | Tomoyuki Nakazawa Department of Pediatrics, Juntendo University Urayasu Hospital 2-1-1 Tomioka, Urayasu, Chiba 279-0021, Japan Tel: +81-47-353-3111 Fax: +81-47-353-0526 E-mail: tnakazawa@juntendo-urayasu.jp |
|
| Received September 11, 2013; Accepted October 26, 2013; Published November 01, 2013 | ||
| Citation: Nakazawa T, Obinata K, Nagata Y, Ebara K, Suzuki K, et al. (2013) A Case of Morganella morganii Meningoencephatitis. J Infect Dis Ther 1:118. doi:10.4172/2332-0877.100011 | ||
| Copyright: © 2013 Nakazawa T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Morganella morganii (M. morganii) is a Gram-negative bacillus found in the environment and among normal human intestinal flora. It is a well known cause of urinary tract infections, wound infections, sepsis and other extra-intestinal infections. It also is considered to be an opportunistic pathogen and has been known to occur both in community and nosocomial infections. Most reported cases of severe infections with M. morganii were in patients with some immunological defects. In this paper, we present a rare case in a child who had a residual cavernous hemangioma in the right frontal lobe and suffered from M. morganii meningoencephalitis.
| Keywords | |
| Meningitis; Morganella morganii; Antimicrobial susceptibility; Cytokines; Nosocomial infections | |
| Introduction | |
| Morganella morganii (M. morganii) is a Gram-negative bacillus that is ubiquitous in the environment. It is also found in the intestinal tracts of humans as normal flora. M. morganii can cause nosocomial and opportunistic infections. However, central nervous system (CNS) infection, such as meningitis due to M. morganii, is very rare. Only 10 cases [1-3] of CNS infection with the bacteria have been reported in the literature. Here, we report a case of meningoencephalitis caused by M. morganii in a 20-month-old boy who had the history of neurosurgical surgery. | |
| Case Report | |
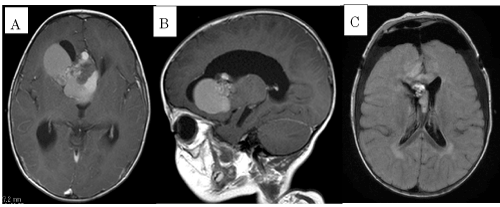
| The patient is a 20-month-old Japanese boy who had no significant history in the perinatal period. At the age of 12-months, he developed appetite loss, vomiting, and unsteadiness. As his general condition gradually worsened, he was referred to our hospital. A cranial MRI showed ventricular dilatation and a mass on the right frontal lobe (Figure 1A and 1B). Hydrocephalus due to a brain tumor was suspected. Immediately, an extradural drainage was performed in order to reduce intracranial pressure. One week later, the tumor was successfully removed by craniotomy in the frontal lobe and the drainage tube was withdrawn. The pathological specimen analysis showed a cavernous hemangioma histologically. The postoperative MRI after the operation showed a residual hemangioma (Figure 1C). Cefazolin was administered for 10 days after the operation and phenobarbital was introduced to prevent seizures. His psychomotor development was within normal range, but sequelar left facial nerve palsy remained. | |
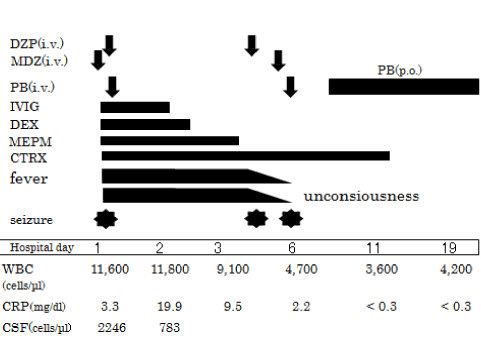
| At the age of 20-months, he experienced a high fever (40°C) and vomiting. A generalized tonic-clonic seizure developed after three hours from the fever onset and spontaneously ceased within five minutes. However, the seizure recurred and he was transferred to our emergency room where the seizure only seized after 15 minutes and midazolam administration. Nevertheless, a generalized tonic-clonic convulsion developed again and that subsided with diazepam and phenobarbital treatment (Figure 2). | |
| Physical examination showed a respiratory rate 44 of breaths/ min, heart rate of 230/min., blood pressure at 116/60 mmHg, and body temperature at 40.3°C. He was consciousness but not fully alert (Glasgow Coma Scale; E4V2M5) and nuchal rigidity was observed. There were no signs of otitis media. | |
| Laboratory studies revealed a slightly elevated White Blood Cell (WBC) count at 11,600 cells/mm3 and elevation of C-reactive protein (CRP) levels at 3.3 mg/dL. The cerebrospinal fluid (CSF) was cloudy with increased white blood cells 2,246/mm3, composed of 72.9% neutrophils, low glucose 25mg/dL and increased protein 202 mg/dL. The cranial CT did not show any signs of hydrocephalus or abscess formation (Figure 1B). The CSF and blood culture grew Gram-negative rods as M. morganii. The organism was resistant to both ampicillin and cefazolin (Table 1). The identification of this bacterium was made by Neg Combo 6.11 panel (Siemens Healthcare Diagnostic Inc.) and antibiotic sensitivity pattern was carried out by MicroScan WalkAway plus System. | |
| He was initially treated with intravenous meropenem (MPEM) 120 mg/kg/day and ceftriaxone (CTRX) 120 mg/kg/day, and subsequently, MPEM was ceased on the third hospital day based on the antimicrobial activity. Intravenous immunoglobulin (1 g/kg/day) and dexamethasone (0.6 mg/kg/2 days) were also administered. A repeat CSF study after 48 hours from the start of antibiotic therapy showed a negative result for the bacteria. On the 4th and 6th hospital day, seizures recurred. The administration of intravenous diazepam, midazolam and phenobarbital successfully stopped the seizures. A lumbar MRI did not show any congenital structural defects. On the EEG, a reduction in spindle formation of the right hemisphere was observed, though epileptic discharges were not present. Auditory Brainstem Response (ABR) was within normal limits. The levels of immunoglobulins (Ig G 780 mg/ dl), subclasses of IgG (IgG1% 62.60, IgG2% 32.26, IgG3% 3.33, IgG4% 1.80), neutrophil functions (sterilizing function 87.3%, phagocytosis function 95.2%), and distribution of T cells (81%) and B cells (9%) were all normal. Cytokines in the CSF at the first lumbar puncture were as follows: IL-6 25,800 pg/mL (normal range <31.2 pg/ml), MMP-9 630 ng/mL (<1.0 ng/ml), TNF-α 26.1 pg/ml (<39 pg/ml), and IFN-γ 5.81 pg/ml (<9 pg/ml). Antibiotic therapy was continued for a total of two weeks. He has appeared well on subsequent follow-ups, except for a mild left hemiparesis of the upper limb and the residual left facial palsy. | |
| Discussion | |
| M. morganii sometimes can cause opportunistic infections. Müller [4] reported that M. morganii was isolated more often from patients with gastrointestinal disease than from healthy controls. It has been reported as a causative agent for pneumonia, empyema, promyositis, endophthalmitis, surgical wound infection, neonatal sepsis, spontaneous bacterial peritonitis [5] and pericarditis [6]. | |
| Lee and Liu [5] reported that 74/10,639 (0.69%) blood cultures were positive for M. morganii during a 2-year-period in a tertiary referral medical center in Taiwan. Most M. morganii bacteremic patients had underlying disorders, such as solid tumors, diabetes mellitus, chronic renal failure, hypertension and non-neoplastic hepatobiliary disease. Portals of entry of M. morganii were the urinary tract (37%), hepatobiliary tract (22%), soft tissue (15%) and the pleuropulmonary system (4.1%). Otogenic infections are also noted as a possible cause of CNS infections by M. morganii [3]. Only one case involving an abscess in the CNS did not have any underlying disease. The mortality rate in this study was 38.3%. Diabetes mellitus, polymicrobial bacteremia and inappropriate treatment were significantly related to the mortality. According to another report, the mortality rate in neonatal M. morganii sepsis was 36% [7]. | |
| While M. morganii has intrinsic resistance to oxacillin, ampicillin, amoxicillin, most of the first- and second-generation cephalosporins and macrolides, it is naturally sensitive to aztreonam, aminoglycosides, antipseudomonal penicillins, third- and fourthgeneration cephalosporins, carbapenems and quinolones. M. morganii can develop resistance to multiple antibiotics by varied mechanisms, such as production of inducible extended-spectrum beta-lactamase [8]. In our case, the detected bacteria were sensitive to CTRX, ceftazidime, ertapenem, imipenem, MEPM, tetracycline, aztreonam and trimethoprim/sulfamethoxazole. | |
| Previous cases of severely infected patients with M. morganii had some immunological defects, such as either a low CD4 lymphocyte count or agammaglobulinemia [2,6,7,9-11]. We describe M. morganii meningoencephalitis in an immunocompetent infant. The patient had a background of neurosurgical operation that did not include a ventriculo-peritoneal shunt. We feel that the possibility of direct invasion of M. morganii into the CNS was negligible. Since M. morganii was isolated from both the CSF and the blood, it may have invaded into the central nervous system hematogenously. We speculate that the use of antibiotics may have caused an imbalance of the enterobacterial flora, resulting in bacterial translocation and bacteremia. | |
| In the present case, the elevation of CSF cytokines was minimal except for IL-6, which may reflect the favorable outcome. We propose that the mild hemiparesis was due to the postoperative complication not to the sequelae of the meningitis. Takasaki et al. [12] reported that the CSF concentrations of MMP-9 in severe cases with purulent meningitis were above 800 ng/mL, while those with a good outcome were below 500 ng/mL. According to Ichiyama et al. [13], both TGF-β1 and TNF-α level in the CSF were important parameters for predicting neurological sequelae in children with bacterial meningitis, though the levels of IL-6 were not a significant factor in their prognosis. | |
| In Japan, cases of bacterial meningitis have diminished dramatically due to the introduction of the Haemophilus influenzae type b (Hib) vaccine since 2008 and the pneumococcal conjugate vaccine since 2010. Now, physicians should consider rare bacterium, such as M. morganii, as a pathogen of bacterial meningitis, not only in immunocompromised hosts and patients with a history of chronic otitis media, but also in patients with a neurosurgical history. | |
References
- Abdalla J, Saad M, Samnani I, Lee P, Moorman J (2006) Central nervous system infection caused by Morganella morganii. Am J Med Sci 331: 44-47.
- Milligan KL, Barenkamp SJ (2013) Neonatal meningitis due to Morganella morganii. Clin Pediatr (Phila) 52: 462-464.
- Patil AB, Nadagir SD, Lakshminarayana S, Syeda FM (2012) Morganella morganii, subspecies morganii, biogroup A: An unusual causative pathogen of brain abscess. J Neurosci Rural Pract 3: 370-372.
- Müller HE (1986) Occurrence and pathogenic role of Morganella-Proteus-Providencia group bacteria in human feces. J Clin Microbiol 23: 404-405.
- Lee IK, Liu JW (2006) Clinical characteristics and risk factors for mortality in Morganella morganii bacteremia. J Microbiol Immunol Infect 39: 328-334.
- Cho YK, Kook H, Woo YJ, Choi YY, Ma JS, et al. (2010) Morganella morganii pericarditis in a child with X-linked agammaglobulinemia. Pediatr Int 52: 489-491.
- Chang HY, Wang SM, Chiu NC, Chung HY, Wang HK (2011) Neonatal Morganella morganii sepsis: A case report and review of the literature. Pediatr Int 53: 121-123.
- Stock I, Wiedemann B (1998) Identification and natural antibiotic susceptibility of Morganella morganii. Diagn Microbiol Infect Dis 30: 153-165.
- Maheshwari A, Dutta S, Kumar P, Narang A (2001) Early onset mixed Morganella and Klebsiella sepsis in a neonate. Indian J Pediatr 68: 671-672.
- Rowen JL, Lopez SM (1998) Morganella morganii early onset sepsis. Pediatr Infect Dis J 17: 1176-1177.
- Ndiaye M, Sène MS, Sow AD, Seck LB, Coulibaly T, et al. (2010) Meningoencephalitis due to Morganella morganii: A case report. Bull Soc Pathol Exot 103: 230-232.
- Takasaki J, Itakura Y, Tamura M (2004) Matrix metalloproteinase-9 measurement in the cerebrospinal fluid of neonates with purulent meningitis. J Jpn Soc Perin Neon Med 40: 813-816.
- Ichiyama T, Hayashi T, Nishikawa M, Furukawa S (1997) Levels of transforming growth factor beta 1, tumor necrosis factor alpha, and interleukin 6 in cerebrospinal fluid: Association with clinical outcome for children with bacterial meningitis. Clin Infect Dis 25: 328-329.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 17103
- [From(publication date):
December-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12516
- PDF downloads : 4587
