A Case of Methylenetetrahydrofolate Reductase Deficiency with Suspected Early-onset Dementia Associated with Hyperhomocysteinemia Due to Reduced Folate
Received: 24-May-2019 / Accepted Date: 05-Jun-2019 / Published Date: 12-Jun-2019 DOI: 10.4172/2161-0460.1000467
Abstract
A 46-year-old woman was admitted for suspected early-onset dementia. At the age of 20 years, she presented with seizures. She was subsequently diagnosed with major depressive disorder and schizophrenia. At age 43, she developed muscular hypotonia and gait disturbance. Three years later, on admission, laboratory studies showed marked hyperhomocysteinemia, hypomethioninemia, and decreased folate level. Brain magnetic resonance imaging revealed multiple cerebral infarction. She received supplements with folate and vitamin B6. After the treatment, serum homocysteine level was reduced but serum folate level was elevated above normal. A diagnosis of methylenetetrahydrofolate reductase (MTHFR) deficiency was considered, so we changed folate to betaine. Sequence analysis of the MTHFR gene demonstrated a missense c.677C>T (p.Ala222Val) mutation. Treatment with betaine resulted in reducing the folic acid level to normal and physical rehabilitation improved the muscular hypotonia. However, psychiatric symptoms such as cognitive impairment and depressive symptoms did not improve during the disease course. This case suggests that measurement of serum levels of homocysteine, methionine, and folate may be useful for identifying the cause of unexplained adult-onset epilepsy, progressive neurological distress, and early-onset multiple cerebral infarction
Keywords: Methylenetetrahydrofolate reductase deficiency; Hyperhomocysteinemia; Multiple cerebral infarction; Betaine; Early-onset dementia
Abbreviations
MTHFR: Methylenetetrahydrofolate Reductase; MMSE: Mini-Mental State Examination; WAIS-III: Wechsler Adult Intelligence Scale-Third Edition; CNS: Central Nervous System
Introduction
Methylenetetrahydrofolate Reductase (MTHFR) is a key enzyme in the folate-dependent remethylation of homocysteine [1]. It reduces 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the predominant circulating form of folate and donor of the methyl group in the remethylation of homocysteine to methionine. MTHFR deficiency is a rare autosomal recessive disease [2], with biochemical features that include homocystinuria, hyperhomocysteinemia, hypomethioninemia, and typically low plasma folate levels [3]. Its morbidity is unclear, but about 300 patients have been reported since its first description in 1972. Its clinical features are highly variable with regard to age of onset and severity and nature of the symptoms, and few cases of adult onset MTHFR deficiency have been described.
Here, we report the case of a 46-year-old woman with MTHFR deficiency and suspected early-onset dementia that was previously diagnosed with major depressive disorder and schizophrenia. The patient provided written informed consent for publication of this report.
Case Presentation
A 46-year-old Japanese woman was referred to our hospital for suspected early-onset dementia based on cognitive impairment and general functional decline. Growth and development in childhood had been normal. She had no history of drug or alcohol abuse or of nutritional deficiency, and no family history of mental disorder. She was nulliparous and her menstrual cycle was regular.
At the age of 20 years, she experienced several episodes of generalized seizures. She was given a diagnosis of epilepsy and was subsequently treated with antiepileptic drugs for 5 years. She later worked as a nursing assistant for 7 years.
At age 39, she was admitted to a psychiatric hospital with a diagnosis of major depressive disorder because of depressive symptoms triggered by financial challenges. After discharge, she had difficulty in finding regular employment and became socially withdrawn. At age 43, she developed muscular hypotonia and gait disturbance and became dependent on crutches. Two years later, she was emergently admitted to hospital because of stuporous state and hypothermia. She was diagnosed as having schizophrenia and was treated with antipsychotic drugs (aripiprazole and paliperidone). Soon after discharge, she was admitted to another hospital because of muscular weakness of all four limbs, upper limb tremor, hypersalivation, and loss of motivation. Reducing the dose of antipsychotic medications resulted in improvement of Extrapyramidal Symptoms (EPS), but cognitive dysfunction was observed. Brain computed tomography imaging showed diffuse atrophy of the cerebral cortex with ischemic changes. She was transferred to our hospital on suspicion of early-onset dementia for further investigation.
On admission, examination revealed dysarthria, EPS, global muscular weakness (left dominant), and gait disturbance. She also displayed psychiatric symptoms such as depressive mood, loss of motivation, lack of vitality, and insomnia. Electrocardiogram and electroencephalogram were normal. Five neuropsychological tests were performed. She scored 25/30 on the Mini-Mental State Examination (MMSE) [4,5] and 21/30 on the revised Hasegawa’s Dementia Scale [6]. Cognitive domains that showed decline on the Neurobehavioral Cognitive Status Examination [7] included attention and visuospatial cognition. Profiles on the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) [8] were full intelligence quotient (IQ) 60, verbal IQ 68, and performance IQ 57. Premorbid IQ was 115, which was estimated using the Japanese Adult Reading Test [9].
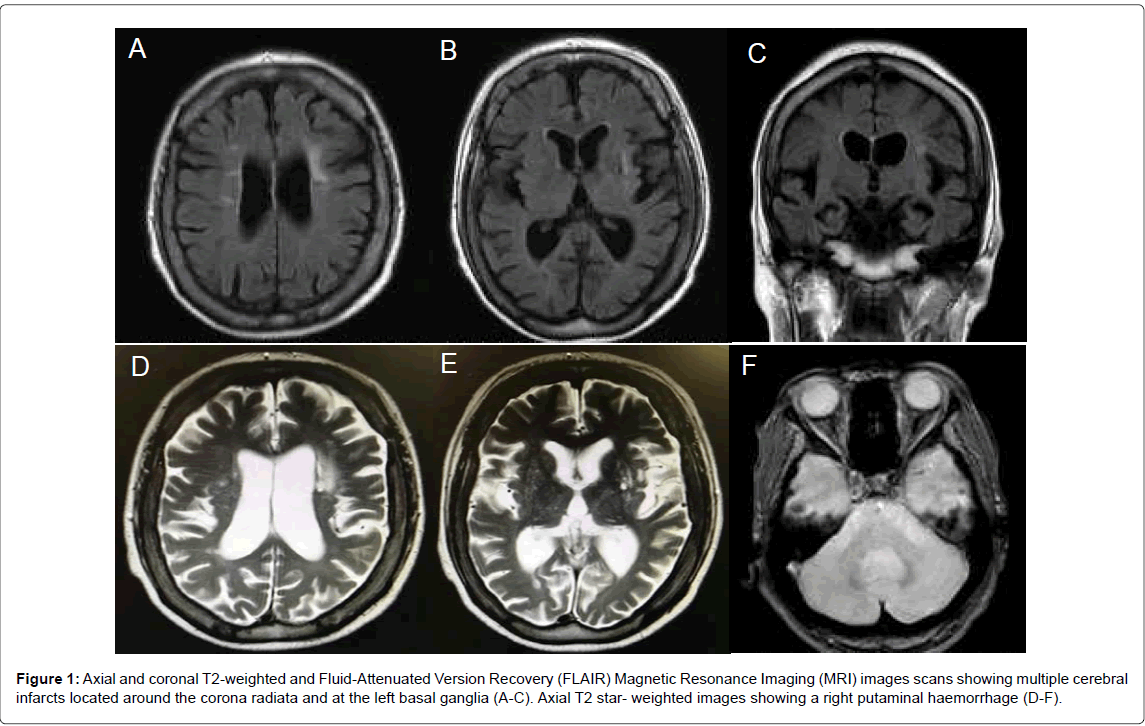
Laboratory evaluation revealed a decreased serum folate level of 1.4 ng/mL (normal range, 3.6-12.9 ng/mL). There was no megaloblastic anemia. Brain magnetic resonance imaging revealed multiple cerebral infarction located around the corona radiate, the left basal ganglia, and the pons on T2-weighted and Fluid-Attenuated Inversion Recovery (FLAIR) images (Figure 1). A right putaminal hemorrhage was detected on T2 star-weighted images.
Figure 1: Axial and coronal T2-weighted and Fluid-Attenuated Version Recovery (FLAIR) Magnetic Resonance Imaging (MRI) images scans showing multiple cerebral infarcts located around the corona radiata and at the left basal ganglia (A-C). Axial T2 star- weighted images showing a right putaminal haemorrhage (D-F).
Additional tests were performed to determine the cause of the multiple cerebral infarction and low serum folate levels. The results were as follows: serum homocysteine, 77.8 nmol/mL (normal, 3.7- 13.5); methionine 16.8 nmol/mL (19.8-32.7), and pyridoxal 3.0 ng/mL (4.0-19.0). These results were suggestive of hyperhomocysteinemia. The patient had no hallucinations or delusions, so the antipsychotics were discontinued. She received folate and vitamin B6 supplements. After the treatment, serum homocysteine level decreased but the serum folate level was elevated above normal (>20 ng/mL). A diagnosis of MTHFR deficiency was considered, so we changed folate to betaine. Treatment with betaine (3,000 mg/day) lowered the folate level to normal and the serum level of homocysteine and methionine also normalized. We consulted our pediatricians and then performed MTHFR gene sequencing. The results showed a 677C>T polymorphism (p.Ala222Val, rs1801133 TT homozygous). MTHFR activity was not measured. Physical rehabilitation improved muscular hypotonia. However, psychiatric symptoms such as cognitive impairment and depressive symptoms did not improve during the disease course.
Discussion
MTHFR deficiency is the most common inborn error of folate metabolism and is a major cause of hereditary homocysteinemia [10]. Disease onset in MTHFR deficiency occurs mostly early in life and adult onset is rare [11]. Patients with severe MTHFR deficiency demonstrate a wide range of clinical symptoms but usually show progressive neurological distress within the two first decades of life [12]. Adolescent or adult-onset MTHFR deficiency may present with mental retardation, motor and gait disturbance, seizures, various psychiatric symptoms, and thrombosis [13]. Seizures are often initially seen in patients with adult-onset MTHFR deficiency [11,14,15] and the epileptic seizures that occurred in our patient at 20 years of age may have been the first clinical manifestation of the illness.
MTHFR deficiency is a severe disease that primarily affects the Central Nervous System (CNS), mainly because of defective myelination [16-18]. Our patient had intermediate hyperhomocysteinemia and multiple cerebral infarctions. Research has indicated that homocysteine can trigger neuronal damage via oxidative stress, DNA damage, and activation of pro-apoptotic factors in cell culture and animal models [19,20]. It is also believed that hyperhomocysteinemia induces endothelial cell damage, reduces vascular compliance, and alters the process of hemostasis [20]. Moreover, homocysteine is a recognized risk factor for atherosclerotic vascular disease and hypercoagulability states [20,21], although a meta-analysis of large unpublished datasets found no association between elevated homocysteine levels and risk of coronary artery disease [22]. Taken together, these findings suggest that hyperhomocysteinemia may have predisposed our patient, at least in part, to developing early-onset multiple cerebral infarctions.
A 677C>T polymorphism in the MTHFR gene as observed in our patient is a common genetic variant that encodes a thermolabile enzyme which is less active at higher temperatures [23]. Individuals who carry two copies of this variant (“TT homozygous”) tend to have reduce enzyme activity with higher homocysteine levels and lower serum folate levels [24].
Treatment approaches in the management of MTHFR deficiency are heterogeneous with regard to selection, combination, and dosage of drugs [11], but betaine is known to be the most effective therapeutic agent [25,26]. The beneficial effect of betaine in severe MTHFR deficiency is mediated through betaine-homocysteine methyltransferase with the use of an alternate methyl donor for remethylation of homocysteine to methionine [17]. Case reports suggest that treatment with betaine may prevent further cognitive decline in surviving symptomatic patients and may prevent cognitive damage and death if treatment is initiated early [14,27,28]. In our patient, biochemical improvement was seen after betaine treatment, but it was not effective for cognitive impairment or depressive symptoms, suggesting that the cognitive damage had become irreversible. We did not use any antidepressant drugs in this case, but they might have had some benefits on depressive symptoms.
MTHFR deficiency is not detected by current routine infantile mass screening testing for congenital amino acid metabolic disease in Japan, since it checks for hypermethioninemia, and the plasma methionine level is usually low or within normal range in affected patients [29]. Unless clinicians maintain a high index of suspicion and are familiar with the various clinical manifestations, this diagnosis is often missed [2]. Thus, because of the need for to start effective treatment as early as possible, MTHFR deficiency should be considered when investigating atypical progressive neurological distress, epilepsy, and cerebral infarction with decreased serum folate.
Conclusion
The fact that severe MTHFR deficiency may occur late in adulthood has strong practical implications. Screening for serum levels of homocysteine, methionine, and folate may be useful for identifying the cause of unexplained adult-onset epilepsy, progressive neurological distress, and early-onset multiple cerebral infarction. Specific treatment should be started early, before irreversible CNS damage occurs.
Conflicts of Interest
Dr. Hori received lecture fees from Eisai Co. Ltd., Pfizer Japan Inc., Novartis Pharma KK, Daiici Sankyo Inc., Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical KK, Yoshitomi Yakuhin Co, Meiji Seika Pharma Co. Ltd., and Mitsubishi Tanabe Pharma Co. All other authors declare no conflict of interest.
Author Contributions
All authors contributed equally to the planning and conduct of the work described in the article. Drs. Katsumura, Sodenaga, and Miyamoto were mainly responsible for drafting this report. Other authors critically checked the drafts.
References
- Kluijtmans LA, Wendel U, Stevens EM, van den Heuvel LP, Trijbels FJ, et al. (1998) Identification of four novel mutations in severe methylenetetrahydrofolate reductase deficiency. Eur J Hum Genet 6: 257-265.
- Iida S, Nakamura M, Asayama S, Kunieda T, Kaneko S, et al. (2017) Rapidly progressive psychotic symptoms triggered by infection in a patient with methylenetetrahydrofolate reductase deficiency: A case report. BMC Neurol 17: 47.
- Arai M, Osaka H (2011) Acute leukoencephalopathy possibly induced by phenytoin intoxication in an adult patient with methylenetetrahydrofolate reductase deficiency. Epilepsia 52: e58-61.
- Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198.
- Kitamura T (1991) Mini-Mental State (MMS) In Otsuka T, Homma A (eds) Assessment of manual of intellectual function for demented elderly (In Japanese). Japan World Planning Co, Ltd., Tokyo 35-38.
- Imai Y, Hasegawa K (1994) The Revised Hasegawa's Dementia Scale (HDS-R)-Evaluation of its usefulness as a screening test for dementia. J Hong Kong Coll Psychiatr 4: 20-24.
- The Northern California Neurobehavioral Group (1995) Neurobehavioral Cognitive Status Examination (COGNISTAT). The Northern California Neurobehavioral Group Inc., California.
- Wechsler D (1997) Wechsler Adult Intelligence Scale. The Psychological Corporation, San Antonio.
- Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y (2006) Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci 60: 332-339.
- Goyette P, Frosst P, Rosenblatt DS, Rozen R (1995) Seven novel mutations in the methylenetetrahydrofolate reductase gene and genotype/phenotype correlations in severe methylenetetrahydrofolate reductase deficiency. Am J Hum Genet 56: 1052-1059.
- Huemer M, Mulder-Bleile R, Burda P, Froese DS, Suormala T, et al. (2016) Clinical pattern, mutations and in vitro residual activity in 33 patients with severe 5, 10 methylenetetrahydrofolate reductase (MTHFR) deficiency. J Inherit Metab Dis 39: 115-124.
- Forges T, Chery C, Audonnet S, Feillet F, Gueant JL (2010) Life-threatening methylenetetrahydrofolate reductase (MTHFR) deficiency with extremely early onset: Characterization of two novel mutations in compound heterozygous patients. Mol Genet Metab 100: 143-148.
- Ogier de Baulny H, Gerard M, Saudubray JM, Zittoun J (1998) Remethylation defects: guidelines for clinical diagnosis and treatment. Eur J Pediatr 157: S77-83.
- Diekman EF, de Koning TJ, Verhoeven-Duif NM, Rovers MM, van Hasselt PM (2014) Survival and psychomotor development with early betaine treatment in patients with severe methylenetetrahydrofolate reductase deficiency. JAMA Neurol 71: 188-194.
- Lossos A, Teltsh O, Milman T, Meiner V, Rozen R, et al. (2014) Severe methylenetetrahydrofolate reductase deficiency: clinical clues to a potentially treatable cause of adult-onset hereditary spastic paraplegia. JAMA Neurol 71: 901-904.
- Surtees R, Leonard J, Austin S (1991) Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet 338: 1550-1554.
- Strauss KA, Morton DH, Puffenberger EG, Hendrickson C, Robinson DL, et al. (2007) Prevention of brain disease from severe 5,10-methylenetetrahydrofolate reductase deficiency. Mol Genet Metab 91: 165-175.
- Kishi T, Kawamura I, Harada Y, Eguchi T, Sakura N, et al. (1994) Effect of betaine on S-adenosylmethionine levels in the cerebrospinal fluid in a patient with methylenetetrahydrofolate reductase deficiency and peripheral neuropathy. J Inherit Metab Dis 17: 560-565.
- Curro M, Gugliandolo A, Gangemi C, Risitano R, Ientile R, et al. (2014) Toxic effects of mildly elevated homocysteine concentrations in neuronal-like cells. Neurochem Res 39: 1485-1495.
- Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14: 6.
- Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P (2014) Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem 29: 339-344.
- Clarke R, Bennett DA, Parish S, Verhoef P, Dotsch-Klerk M, et al. (2012) Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med 9: e1001177.
- Dean L (2012) Methylenetetrahydrofolate Reductase Deficiency. National Center for Biotechnology Information, Medical Genetics Summaries, Bethesda.
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111-113.
- Michot JM, Sedel F, Giraudier S, Smiejan JM,Papo T (2008) Psychosis, paraplegia and coma revealing methylenetetrahydrofolate reductase deficiency in a 56-year-old woman. J Neurol Neurosurg Psychiatry 79: 963-964.
- Haworth JC, Dilling LA, Surtees RA, Seargeant LE, Lue-Shing H, et al. (1993) Symptomatic and asymptomatic methylenetetrahydrofolate reductase deficiency in two adult brothers. Am J Med Genet 45: 572-576
- Wendel U, Bremer HJ (1984) Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur J Pediatr 142: 147-150.
- Holme E, Kjellman B, Ronge E (1989) Betaine for treatment of homocystinuria caused by methylenetetrahydrofolate reductase deficiency. Arch Dis Child 64: 1061-1064.
- Yuasa N, Ishikawa T, Tokuoka K, Kitagawa Y, Takagi S (2008) Case of juvenile stroke caused by methylenetetrahydrofolate reductase deficiency. Rinsho Shinkeigaku 48: 422-425.
Citation: Katsumura K, Sodenaga M, Miyamoto S, Sasaki O, Akiyama T, et al. (2019) A Case of Methylenetetrahydrofolate Reductase Deficiency with Suspected Early-onset Dementia Associated with Hyperhomocysteinemia Due to Reduced Folate. J Alzheimers Dis Parkinsonism 9:467. DOI: 10.4172/2161-0460.1000467
Copyright: © 2019 Katsumura K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3683
- [From(publication date): 0-2019 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2905
- PDF downloads: 778