A Case of Exophytic Type 2A Papillary Renal Cell Carcinoma with Massive Necrosis
Received: 13-Nov-2015 / Accepted Date: 01-Jan-2016 / Published Date: 04-Jan-2016 DOI: 10.4172/2161-0681.1000263
Abstract
Most renal cell carcinomas (RCCs) are clear cell RCCs, the second most common being papillary RCCs (PRCCs). PRCCs are sub classified as type 1, type 2A, type 2B and mixed type. Pure type 2A PRCCs are estimated to account for only approximately 1-2% of malignant tumors of the kidney. In this paper, we show a case of an exophytic type 2A PRCC with massive necrosis arising in an 80-year-old male. The tumor, which measured 45mm in diameter and showed exophytic growth, was in the left kidney. The tumor was resected by a partial resection of the kidney and was found to be mainly necrotic, while the viable part showed papillary growth. There were small cuboidal cells with eosinophilic cytoplasm covering thin papillae with a single line of uniform small-to-medium sized nuclei and small nucleoli. The tumor was also positive for cytokeratin 7. Following Yang et al, we diagnosed the tumor as a type 2A PRCC.
Conclusion: Type 2 PRCCs should be clearly differentially diagnosed into type 2A or 2B, since the prognosis is different between these two types.
Keywords: Papillary renal cell carcinoma; Type 2A; Exophytic growth; Massive necrosis
312806Introduction
Most renal cell carcinomas (RCCs) are clear cell RCCs. Although papillary renal cell carcinomas (PRCCs) are the second most frequent malignant neoplasia in the kidney, they account worldwide for only 10-15% of all renal carcinomas [1]. PRCCs are histologically characterized by the presence of fibrovascular cores with tumor cells arranged in papillary or tubulopapillary architecture [2]. In Japanese patients, the incidence of PRCCs is lower, or only approximately 5-6% of RCCs [3,4]. PRCCs were first reported by Mancilla-Jimenez et al. in 1976 [5]. PRCCs have been reported to have a better prognosis than clear cell RCCs [5,6]. Delahut and Eble proposed a subclassification of PRCCs into type 1 and type 2 tumors, based on their histological features [7]. In their classification, type 1 PRCCs consist of papillae and tubular structures covered by small cells with pale cytoplasm and characterized by small oval nuclei with inconspicuous nucleoli, frequent glomeruloid papillae, papillary edema, foamy macrophages in papillary cores, and psammoma bodies. In contrast, type 2 PRCCs consist of papillae covered by large cells with abundant eosinophilic cytoplasm and characterized by pseudostratification and large spherical nuclei with prominent nucleoli. The differences between types 1 and 2 are both morphological and clinical. It has been postulated that type 2 PRCCs are associated with a significantly higher Fuhrman grade and poorer prognosis than type 1 PRCCs [8,9].
Yang et al. further sub classified type 2 PRCCs into types 2A and 2B. Type 2A PRCCs show a low Fuhrman nuclear grade despite pleomorphic nuclei, while type 2B shows a high Fuhrman nuclear grade with pleomorphic nuclei [10]. It is interesting that type 2A PRCCs are associated with a better prognosis, similar to type 1 PRCCs, while type 2B PRCCs are associated with a poorer prognosis. Yang et al. also suggested that pure type 2A PRCCs are rare, accounting for only approximately 12% (4/34 cases) of PRCCs. Therefore, type 2A PRCCs are estimated to represent only approximately 1-2% of renal malignant neoplasia.
In this paper, we present a case of exophytic type 2A PRCC with massive necrosis arising in an 80-year-old male.
Case report
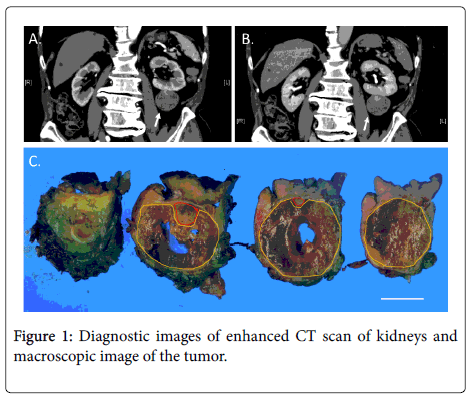
An 80-year-old male consulted our hospital about a tumor in the left kidney that had been discovered incidentally, during a follow-up ultrasound examination for gastroesophageal reflux. The patient had hypertension, chronic cerebral ischemia and gastroesophageal reflux. An abdominal CT scan revealed an approximately 45 mm-diameter tumor with exophytic growth from the inferior pole of the left kidney without enlarged lymph nodes or metastatic lesions. The contrastenhanced CT scan revealed that a significant part of the tumor was not enhanced and a part of the tumor was enhanced in a delayed phase (Figures 1A and B). The non-enhanced area was assumed to show fluid or necrosis, while the delayed-enhanced area was assumed to show viable tumor cells.
A and B: Enhanced CT scan was carried out for the diagnosis of the kidney tumor. The images of early phase (A) and late phase (B) of the enhanced CT scan are shown. The arrows show the tumor; C: Macroscopical images of inside of the tumor are shown. Bar=20mm. Yellow lines circumscribed the necrotic areas, while red lines circumscribe the areas, that contained viable tumor cells.
CT scan revealed that a significant part of the tumor was not enhanced and a part of the tumor was enhanced in a delayed phase. The non-enhanced area was assumed to show fluid or necrosis, while delayed-enhanced area was assumed to show viable tumor cells. The resected tumor was almost spherical with a diameter of approximately 45 mm. The tumor had a capsule and the necrotic area occupied approximately 90% of the tumor.
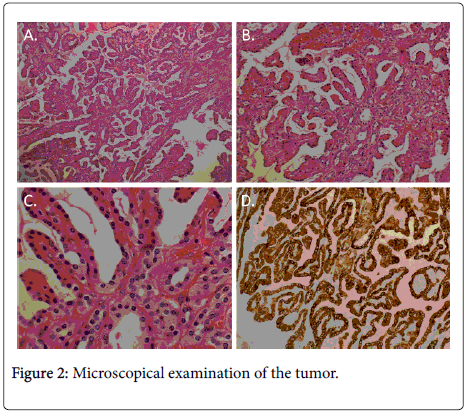
The kidney was partially resected to remove the tumor, including the fatty tissue surrounding the kidney. The removed specimen weighed 92.9 g. Morphologically, the tumor was almost spherical, with a diameter of approximately 45 mm. The tumor had a capsule and, macroscopically, we found most of the tissue inside the capsule to be necrotic, suggesting tumor necrosis (Figure 1C). Histologically, the tumor was encapsulated by fibrous tissue. Even under microscopical examination, we found that most of the tumor (approximately 90% of the tumor) was necrotic, though viable tumor cells were found at the cranial side (the kidney side) of the tumor. The viable tumor cells were either attached to the capsule or adjacent to the capsule. The tumor showed a papillary structure with a fibrovascular core (Figures 2A-2C). This consisted of small cuboidal cells with an eosinophilic cytoplasm covering thin papillae with a single line of uniform nuclei (Fuhrman grade 2) and small nucleoli. The tumor cells immunohistochemically expressed cytokeratin 7 (CK7), partially expressed P504S (AMACR), and did not express CD10, RCC or P53 (Figure 2D and data not shown). Therefore, we diagnosed the tumor as a type 2A PRCC, as described by Yang et al. [10].
In the 6 months (to date) since the resection of the PRCC, the patient has shown no signs of recurrence.
Discussion
In this paper, we have presented a case of type 2A PRCC. Although patients with PRCCs often present with hematuria, abdominal pain and abdominal mass, some PRCCs have been discovered incidentally [11,12]. In our case, the patient did not show any symptoms related to the tumor, which was incidentally found on follow-up ultrasound examination for gastroesophageal reflux. It has been reported that PRCCs form well-circumscribed masses and frequently have thick fibrous capsules or pseudocapsules [13], which is consistent with our case. It has also been reported that small PRCCs are usually solid but that large PRCCs frequently show cystic change, and that hemorrhage and necrosis are frequently seen in PRCCs [13]. Clear cell RCCs usually show a hypervascular pattern in the early phase upon angiography, suggesting that clear cell RCCs have an abundant flow of blood. However, PRCCs show a delayed-enhanced pattern, suggesting that the blood flow is lower than in clear cell RCCs. Therefore, ischemia of the tumor resulting in necrosis could easily occur in PRCCs. The reported mean tumor sizes and range of sizes were 6.7 cm and 1.8-18 cm, respectively, in a study by Amin et al. and 7.2cm and 1.2-26 cm, respectively, in a study by Delahunt et al [7,13]. Although, at 4.5 cm in diameter, the PRCC in our case was smaller than the mean size of reported PRCCs, our PRCC had massive necrosis. The exophytic growth in our case could have accelerated the loss of blood flow in the tumor, resulting in the induction of necrosis by ischemia. From our case, we suspect that tumor necrosis could initially occur in PRCCs, and that the spaces left after the absorption of the necrotic tissue could become cysts.
A: A microscopical image of the tumor upon H and E staining (original magnification of the objective lens: X2); B: A microscopical image of the tumor upon H and E staining (original magnification of the objective lens: X20); C: A microscopical image of the tumor upon H and E staining (original magnification of the objective lens: X60); D: Immunohistochemical examination for CK-7 expression in the tumor. The specimen was stained with anti-CK-7 antibody (original magnification of the objective lens: X20).
The tumor showed a papillary structure with a fibrovascular core. This consisted of small cuboidal cells with an eosinophilic cytoplasm covering thin papillae with a single line of uniform nuclei (Fuhrman grade 2) and small nucleoli. The tumor cells immunohistochemically expressed cytokeratin 7 (CK7).
Histologically, our case was consistent with a type 2A PRCC, as described in the “Case report” section. Yang et al. also reported that types 1 and 2A PRCCs usually express cytokeratin 7 (CK7), but that type 2B PRCCs usually do not [10]. Our case the PRCC also expressed CK7, meaning that it was consistent with a type 2A PRCC based on not only morphology but also immunohistochemically. CK7 is a marker of the distal renal tube and collecting duct, suggesting that types 2A and 2B could have different origins and that typ2A PRCCs might originated from distal tubules, since RCCs originated from the collecting duct have been clearly subclassified from other subtypes of RCCs. Yang et al. also reported that the gene expression profiles of type 2A PRCCs were different from those of type 2B PRCCs [10], suggesting the promising future development of individualized therapy for each subtype.
Conclusion
In this paper, we have reported on a type 2A PRCC with massive necrosis. Type 2 PRCCs should be clearly differentially diagnosed into type 2A or 2B, since the prognosis is different between these two types. Our case also suggests the mechanism underlying cyst formation in PRCCs.
Acknowledgements
We thank Ms K. Ando (Department of Stem Cell Disorders, Kansai Medical University) and Mr. Hilary Eastwick-Field for preparation of this manuscript. We also thank Ms. H. Ogaki, Mr. K Nagaoka, Mr. T. Kuge, Mr. H. Takenaka and Ms. S. Kawasaki at Toyooka Hospital for their expert technical assistance. The authors declare that they have no conflicts of interest. The patient provided informed consent for the publication, and the ethics committee also approved the publication of this case report.
References
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, et al. (1997) The Heidelberg classification of renal cell tumours. J Pathol 183: 131-133.
- Kovacs G (1989) Papillary renal cell carcinoma. A morphologic and cytogenetic study of 11 cases. Am J Pathol 134: 27-34.
- Yamashita S, Ioritani N, Oikawa K, Aizawa M, Endoh M, et al. (2007) Morphological subtyping of papillary renal cell carcinoma: clinicopathological characteristics and prognosis. Int J Urol 14: 679-683.
- Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, et al. (2005) Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. EurUrol 48: 593-600.
- Mancilla-Jimenez R, Stanley RJ, Blath RA (1976) Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer 38: 2469-2480.
- Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, et al. (2002) Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J SurgPathol 26: 281-291.
- Delahunt B, Eble JN (1997) Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 10: 537-544.
- Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, et al. (2001) Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol 32: 590-595.
- Mejean A, Hopirtean V, Bazin JP, Larousserie F, Benoit H, et al. (2003) Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol 170: 764-767.
- Yang XJ, Tan MH, Kim HL, Ditlev JA, Betten MW, et al. (2005) A molecular classification of papillary renal cell carcinoma. Cancer Res 65: 5628-5637.
- Kuroda N, Toi M, Hiroi M, Enzan H (2003) Review of papillary renal cell carcinoma with focus on clinical and pathobiological aspects. HistolHistopathol 18: 487-494.
- Mydlo JH, Bard RH (1987) Analysis of papillary renal adenocarcinoma. Urology 30: 529-534.
- Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, et al. (1997) Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J SurgPathol 21: 621-635.
Citation: Magaribuchi T, Adachi Y, Kuroda N, Sakatani T, Taki Y, et al. (2016) A Case of Exophytic Type 2A Papillary Renal Cell Carcinoma with Massive Necrosis. J Clin Exp Pathol 6:263. DOI: 10.4172/2161-0681.1000263
Copyright: © 2016 Magaribuchi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12010
- [From(publication date): 2-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 11104
- PDF downloads: 906