Research Article Open Access
A 2-year Double-Blind RCT Follow-up Study with Fermented Papaya Preparation (FPP) Modulating Key Markers in Middle-Age Subjects with Clustered Neurodegenerative Disease-Risk Factors
Francesco Marotta1*, Massimiliano Marcellino1, Umberto Solimene2, Biagio Cuffari3, Hariom Yadav4, Alexander N Khokhlov5, Aldo Lorenzetti1, Amelie Mantello6, Joseph Cervi1 and Roberto Catanzaro31ReGenera Research Group for Aging Intervention, San Babila Clinic, Milano, Italy
2WHO-Center for Traditional Medicine and Biotechnology, University of Milano, Italy
3Department of Internal Medicine, University of Catania, Catania, Italy
4Center for Diabetes, Obesity and Metabolism, Wake Forest Medical Center, Biotech Place, Winston-Salem, USA
5Evolutionary Cytogerontology Sector, School of Biology, Moscow State University, Moscow, Russia
6Osato Research Institute and Labs, Gifu, Japan
- *Corresponding Author:
- Prof. Francesco Marotta
ReGenera Research Group for Aging Intervention
San Babila Clinic, Corso Matteotti 1/A
20121 Milano, Italy
Tel: +39-024077243
E-mail: fmarchimede@libero.it
Received date: April 28, 2017; Accepted date: May 16, 2017; Published date: May 18, 2017
Citation: Marotta F, Marcellino M, Solimene U, Cuffari B, Yadav H, et al. (2017) A 2-year Double-Blind RCT Follow-up Study with Fermented Papaya Preparation (FPP) Modulating Key Markers in Middle-Age Subjects with Clustered Neurodegenerative Disease-Risk Factors. Clin Pharmacol Biopharm 6:170. doi: 10.4172/2167-065X.1000170
Copyright: © 2017 Marotta F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
In recent years a number of studies have reported the significant relationship between metabolic syndrome and neurodegenerative disease. There is accumulating evidence that the interplay of combined genetic and environmental risk factors (from diet to life style to pollutants) to intrinsic age-related oxi-inflammatory changes may be advocated for to explain the pandemic of neurodegenerative diseases. In recent years a specific Fermented Papaya Preparation (FPP) has been shown to significantly affect a number of redox signalling abnormalities in a variety of chronic diseases and as well in aging mechanisms either on experimental and on clinical ground. The aim of the present study was to evaluate FPP use in impending metabolic disease patients with potentially neurodegenerative disease clustered risk factors. The study population consisted of 90 patients aged 45-65 years old, with impending metabolic syndrome and previously selected as to be ApoE4 genotype negative. By applying a RCT, double-blind method, one group received FPP 4.5 g twice a day (the most common dosage utilized in prior clinical studies) while the other received an oral antioxidant cocktail (trans-resveratrol, selenium, vitamin E, vitamin C). Then, after 21 month treatment period, a selected heavy metal chelator was added at the dosage of 3 g/nocte for the final 3 months study treatment. The parameters tested were: routine tests oxidized LDL-cholesterol, anti-oxidised LDL, Cyclophilin-A (CyPA), plasminogen activator inhibitor-1 and CyPA gene expression. From this study it would appear that FPP, unlike the control antioxidant, significantly decreased oxidized-LDL and near normalizing the anti- Ox-LDL/Ox-LDL ratio (p<0.001) although unaffecting the lipid profile per sè. Moreover, only FPP decreased cyclophilin-A plasma level and plasminogen activator-inhibitor (p<0.01) together with downregulating cyclophilin-A gene expression (p<0.01). Insulin resistance was only mildly improved. Heavy metals gut clearance proved to be effectively enhanced by the chelator (p<0.01) and this was not affected by any of the nutraceuticals, nor it added any further benefit to the biological action of FPP.
Keywords
Fermented Papaya Preparation (FPP); Redox balance; Antioxidant; Neurodegenerative disease; Caviarlieri
Introduction
Starting in the early 90’ gold standard electron spin resonance studies had shown that a functional food consisting of fermented papaya (FPP, ORI, Oxidative Stress laboratory, Gifu, Japan) exhibited a powerful anti-oxidative activity on in vitro cerebral cells [1] as well on in vivo epilepsy experimental model, where the epileptogenic monoamine neural release was consistently reduced [2]. Later on, a Japanese group proved the capacity of FPP to reduce the derangement of oxidant/antioxidant balance at the brain level in elderly rats and in experimental ischemia-reperfusion induced cerebral damage as well [3,4]. In recent years our group has shown that FPP could beneficially effect on clinical ground a number of redox signaling abnormalities in a variety of chronic diseases [5,6] as well as some key aging mechanisms [7,8].
Recent studies using transgenic mice have shown the importance of proinflammatory Cyclophilin A (CypA)-metalloproteinase-9 in bloodbrain- barrier integrity [9] while other have proved that a specific inhibitor of CyPA could reduce neuroinflammation, improve motor neurons activity and prolong survival in a mouse model of amyotrophic lateral sclerosis [10]. The potential neuroprotective effects of FPP are at the moment the target of a clinical study on Parkinson’s disease patients by the neurology group of Nordera. This group has reported some preliminary promising results such as a reduction of motor scores of the Unified Parkinson Disease Rating Scale, improvement of Activity of Daily Living performance and of redox biochemistry (G. Nordera, personal communication at Oxidative Stress in Health and Prevention, September 24, 2014 http://www.centrostressossidativo.it/video-congresso-stress-ossidativo/). This latter aspect has received further support by the fine biochemical analysis of same patients cohort by Bolner et al. [11]. Moreover, it has been recently shown that FPP could dramatically decrease the oxidative stress parameters in established Alzheimer Disease (AD) patients [12].
In recent years a number of studies have reported the significant relationship between metabolic syndrome and neurodegenerative disease [13-15]. Indeed, current views on dietary-related diseases and also neuroimaging data have reported the association between dismetabolic patterns and neurodegenerative damage [16] where lowgrade inflammation and insulin-resistance seem to be among main background factors involved [17-19]. As for metabolic syndrome, different groupings of the following metabolic parameters have been listed in each classification, such as insulin resistance, hypertriglyceridemia, low HDL-C, obesity/increased waist circumference, altered glucose tolerance/diabetes mellitus, hyperinsulinemia, microalbuminuria and hypertension. In the current study we referred to the definition of MS as outlined by the American Heart Association and the National Heart, Lung, and Blood Institute [20].
A further open issue regarding the multifactorial pathophysiology of neurodegenerative disease is the potentially detrimental role played by occupational and environmental exposure to heavy metals [21-25]. Indeed, while some metals are involved in physiological enzymatic reactions, once they accumulate in the brain, especially if overtly acting as xenobiotic, may give raise to oxidative stress phenomena with mitochondrial dysfunction, protein misfolding and aberrant autophagic processes [26-28]. As a matter of fact the update literature suggests that the aggregation of disease-related proteins during physiological aging can be advocated for by abnormal protein homeostasis observed. Such abnormalities may thus represent a biomarker of aging that could modulate life span and cause neurodegeneration. Albeit not fully unfolded as yet, it seems that the interplay between combined genetic and environmental risk factors (from diet to life style to pollutants) with intrinsic age-related oxiinflammatory changes may be advocated for to explain the current increasing surge of neurodegenerative diseases. Given all above, the current challenging quest of medical community is mostly focused to track down early biomarkers of likely modifiable causative factors together with possible beneficial approaches within a multifunctional therapeutic armory. The aim of the present study was to evaluate FPP use in impending metabolic disease patients with potentially higher risk to develop a neurodegenerative disease.
Methods
Ethics
All procedures were approved by an independent Ethical Committee for nonpharmacological research (ReGenera Research Group for Aging Intervention, protocol FPP-MSNEU-10/2015). Each subject recruited for the study was fully informed and treated in compliance with the guidelines of the Declaration of Helsinki.
FPP
The FPP used in the present study was obtained from Carica papaya L. cultivated in Hawaii, following yeast fermentation for 10 months and pharmaceutical-grade batch-to-batch control at the Osato Research Institute (Gifu, Japan). The final composition of FPP per 100 g is as follows: 90.7 g carbohydrates, 17 μg vitamin B6, 2 μg folic acid, 2.5 mg calcium, 16.9 mg potassium, 240 μg niacin, 4.6 mg magnesium, 14 μg copper, 75 μg zinc, 16 mg arginine, 6 mg lysine, 5 mg histidine, 11 mg phenylalanine, 9 mg tyrosine, 18 mg leucine, 9 mg isoleucine, 5 mg methionine, 13 mg valine, 11 mg glycine, 8 mg proline, 37 mg glutamic acid, 11 mg serine, 8 mg threonine, 27 mg aspartic acid, and 2 mg tryptophan. This formulation was chosen also to replicate a similar mixture reported on the market to have antioxidant effect in humans. Other claimed “fermented papaya” extracts available on the Italian market were excluded since prior in-house electron spin resonance testing had ascertained their poor to nil antioxidant effect (Mantello P. ORI Oxidative Stress Research Lab, Gifu, Japan. In-house data).
Study design
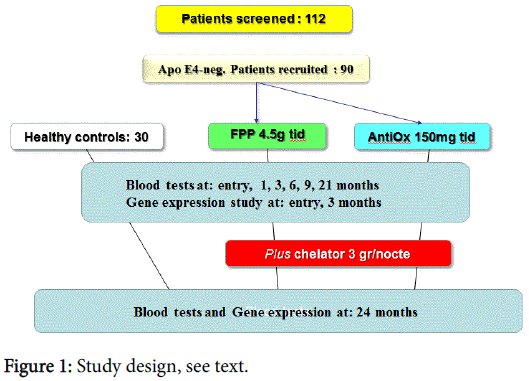
The study population consisted 90 patients ranging from 45 to 65 years old, with impending metabolic syndrome (2 parameters out of the above-mentioned classification) and 30 age-matched healthy volunteers (Table 1). Patients with secondary hypertension, cardiomyopathy, severe abnormalities of liver, thyroid and kidney function, cerebrovascular diseases, grossly elevated total cholesterol (>280 mg/dl or LDL >180 mg/dl), malignancies and history of coronary bypass surgery or on insulin treatment were excluded from this study. Exclusion criteria were also the consumption of antioxidant or other supplements in the past 3 months.
| Parameters | Healthy Control | FPP-treated | Antioxidant-treated |
|---|---|---|---|
| Total no. (male/female) | 30 (15/15) | 45 (33/18) | 45 (28/17) |
| Mean age | 67 | 71 | 66 |
| Mean BMI (range) | 23 (21-25) | 27 (23-35) | 26 (24-34) |
| Family history of diabetes | 1/30 | 14/45 | 21/45 |
| Family history of hypertension | 1/30 | 17/45 | 18/45 |
| Overt diabetes | NA | 6/45 | 11/45 |
| Duration of diabetes (years) | NA | 4 | 3 |
| Dyslipidemia | 0/30 | 28/45 | 31/45 |
| Smoking | 4/30 | 7/45 | 12/45 |
| Waist (cm) | 82 | 98 | 99 |
| Waist/hip ratio | 0.82 | 0.92 | 0.95 |
| Mean Systolic Blood Pressure (mmHg) | 122 | 159 | 161 |
| Mean Diastolic Blood Pressure (mmHg) | 65 | 78 | 81 |
| Physical activity | 15/30 | 6/45 | 12/45 |
Table 1: Anthropometric, clinical and biological parameters in studied subjects.
All patients were screened for ApoE4 polymorphism to rule out the presence of a known risk gene pattern for neurodegenerative disease. This was a RCT, double-blind study, with FPP 4.5 g given twice a day vs. common antioxidant cocktail (trans-resveratrol 20 mg, selenium 60 mcg, vit E 30 mg, vit C 100 mg, papaya flavour) twice a day. Then, after 21 month continuous treatment period, an heavy metal chelator (talcsize, higher potency chabasite-phillipsite zeolites naturally-occurring mixture, Novagenics-SOL Ltd., Hong Kong) was added at the dosage of 3 g/nocte for further 3 months to complete the 24 months study (Figure 1).
At entry 1, 3, 6, 12, 21 and 24 months, biological samples were collected (BD Vacutainer sampling tubes) to assess biochemical assessments as below mentioned. Blood serum was isolated by centrifugation and stored at under −70°C until analysis. Repeated thawing and freezing were avoided.
Parameters
Pre-recruiting gene testing: Ten millilitres of peripheral whole blood was withdrawn from an antecubital vein of all recruited subjects. Genomic DNA was extracted by a modified salting-out method (by precipitating DNA with the use of 96% ethanol). This was processed to ApoE genotyping with modifications in the multiplex amplification refractory mutation system PCR (Multi-ARMS PCR). The primer sequences used in the PCR analysis were as follows: APOE rs7412 F:ATGCCGATGACCTGCAGA R:ACTGGCGCTGCATGTCTT Product size (bp) 684; rs429358 F:TCGGAACTGGAGGAACAACT R:TACACTGCCAGGCGCTTCT Product size (bp) 260. Genomic DNA (50 ng) amplification (ABI Prism 310, Perkin Elmer, USA) comprised 30 cycles (94ºC for 5 min, 94ºC for 30 s, 65ºC for 30 s, and 72º for 30 s), followed by a cycle of 10 min at 72°C for the final chain extension. Moreover, for the analysis of restriction fragment length polymorphism, the amplification product was digested with HhaI, followed by 5.0% agarose gel electrophoresis for 2 h and 30 min, stained with electro-fluorescent red stain (ThermoFisher, USA) and UV light-visualization of the DNA fragments. As stated above, ApoE4 subjects were excluded from the study.
Biochemical tests: routine tests (WBC, transaminases, urea, creatinine, glucose, glycated haemoglobin, uric acid, HOMA test, LDL and HDL cholesterol) were assayed by using the 7060 Automatic Biochemical Analyzer (Hitachi, Ltd, Tokyo, Japan). Oxidized cholesterol, anti-oxidised LDL, ratio of anti-ox-LDL/ox-LDL cyclophilin-A, plasminogen activator inhibitor-1 were tested as below.
Oxidised LDL and anti-oxidised LDL antibodies assessment: These parameters were obtained by fractionation of LDL and preparation of oxLDL as antigen in the antibody detection assay, according to what reported by Ketelhuth et al. [29]. For this purpose a commercially available ELISA kits (ChusaBiotech Co. Ltd., China), was employed. Briefly, microtiter plates were coated with 10 g/ml in PBS of either native LDL or MDA-LDL to measure anti-oxidized LDL antibodies. Serum samples were diluted 1:100 in 1% BSA/PBS and incubated for 2 h at 37°C and overnight at 4 C. Afterwards, the plates were washed four times with alkaline phosphatase-conjugated anti-human IgG (Sigma Chemical, St. Louis, MO) and left for 3 h at room temperature. The reaction was halted after 60 min with 1% BSA/PBS for 2 h at room temperature. The absorbance was at 450 nm using a Multiskan RC ELISA reader (Thermo Fisher Scientific, USA). The binding of antibodies to oxidized LDL was determined by subtracting native LDL binding value from the one of MDA-LDL while the results were expressed as an Optical Density (OD).
Measurement of cyclophilin A in plasma: Cyclophilin A concentration in plasma was assayed with quantitative sandwich enzyme immunoassay kit (Wouhan-UCSN BioScience, Wouhan, China). Briefly, a monoclonal antibody specific for CypA was precoated onto a microplate. Standards and samples were put into the wells and CypA present is bound by the immobilized antibody. An enzyme-linked polyclonal antibody specific for CypA is added to the wells. Plates were washed three times with PBS and a substrate solution was added to the wells. The following dose-dependent color appearance was then halted by an acid solution and the color intensity measured by a reader as above. There was no evidence of any detectable cross-reactivity with any other tested proteins. All samples were analyzed in triplicate and samples with a CV >10% were discarded.
Determination of plasminogen activator inhibitor-1: PAI-1 serum concentration was measured by sandwich enzyme ELISA (PAI-1; DSE100; R&D Systems). Active PAI-1 levels were calculated from a standard curve constructed by using recombinant human PAI-1. By this method either active or latent forms of PAI-1 are detected [30]. Intra and inter-assay coefficients of variation of PAI-1 antigen were 3.9% and 4.8%, respectively.
Cyclophilin-A gene expression study: Reverse transcriptionpolymerase chain reaction (RTPCR) was used to determine their expression at 0, 90 days and at the end of the 24 month study period. Briefly, cells were lysed by Ambion lysis buffer (Ambion, Carlsbad, CA, USA) for 20 min and the lysates were mixed with same volume of 64% ethanol. The lysate products were put into a column and centrifuged at 10,000 × g for 1 min. Afterwards, a buffer consisting of 700 μl buffer 1 and 500 μl buffer 2/3 (Genosolution, Ltd., Shenyang, China) was used to wash the column. After incubation with 50 μl buffer (GenePharma Co., Ltd.), the resulting flow was collected and 50 μl elution buffer was added and a further centrifugation was performed. Next, 1 μl DNAse I was added to 20 μl of RNA solution with suitable DNAse I buffer (GenePharma Co., Ltd.) and incubated at 37Ë?C for 2 h. The DNAse I was removed using DNAse reagent and purified RNA obtained by a centrifugation at 10,000 × g for 1 min. The quantity of total RNA was measured by optical density at 260 nm and the procedure checked through 1.5% agarose gel electrophoresis. RT was run in a 20ml solution containing 3 μg total RNA using the Revert cDNA Synthesis kit (Toyobo Biotech, Co., Ltd., Shanghai China). PCR was done in a thermal cycler after prior denaturation at 94Ë?C for 5 min, followed by amplification for 30 cycles of denaturation at 94Ë?C for 40 s, annealing phase at 65Ë?C for 1 min followed by 72Ë?C for 1 min. Afterwards, 5 μl PCR material was isolated by electrophoresis on a 1.5% agarose gel and stained by ethidium bromide. The following primer was used to carry out the test: CypA forward, 5'GTC AAC CCC ACC GTG TTC TTC3', and reverse, 5'TTT CTG CTG TCT TTG GGA CCTTG3' and glyceraldehyde3phosphate dehydrogenase (GAPDH) forward, 5'ACC ACA GTC CAT GCC ATCAC3', and reverse, 5'TCC ACC ACC CTG TTG CTGTA3'. The PCR products were subjected to electrophoresis on a 1.4% agarose gel and the results were quantitatively assessed by Gelmatrix Scan software (BioMatrixscan Inc., Shanghai, China).
Faecal heavy metal measurement
A 15 mg of stool sample was collected and an inductively coupled argon plasma mass spectrometry (ICP-MS) with an Agilent 7500ce device was used to test the following heavy metals: lead, arsenic, mercury, cadmium, nickel and beryllium. According to the IUPAC guideline [31] the reference value was defined within the 95% confidence interval of the 95th population percentile. The results in the specimens were expressed as μg/L. Data were expressed on a dry weight basis so to avoid the variability related to watery component of the sample.
Results
Routine tests
Blood tests (WBC, creatinine, urea, glucose, glycated haemoglobin, uric acid, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, transaminases, gamma-GT, alkaline phosphatase, bilirubin) were not affected by any of the nutraceuticals employed, except a not significant trend improvement of HOMA test in the FPPsupplemented group (data not shown).
Oxidized LDL cholesterol and anti-oxidised LDL antibodies
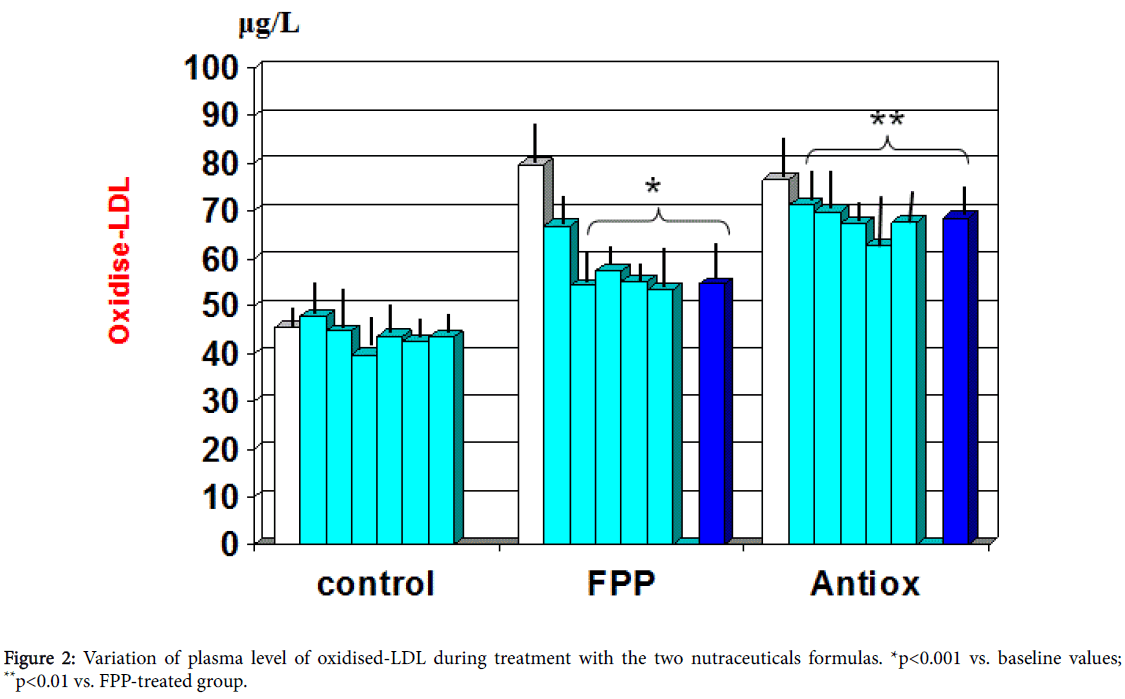
Whereas the group treated with antioxidant cocktail did not show any statistically significant difference in the oxidised lipid asset, patients treated with FPP showed a significant improvement of these parameters (Figure 2). In particular, the oxidized LDL cholesterol was normalised by this intervention (p<0.001 vs. baseline). This variation was significantly detectable starting from the third month observation period while the anti-Ox-LDL reverted to near to normal level from 12 months observation time onwards (p<0.05, Table 2). There was no significant correlation between these parameter and main clinical history data (presence or duration of diabetes, hypertension or dyslipidemia). No significant gender difference was detected as for these parameters are concerned.
| Anti-ox-LDL EU/mL (18.2 ± 3.4) |
Entry | 6 months | 12 months | 21 months | 24 months |
|---|---|---|---|---|---|
| FPP | 28.1 ± 10.2 | 28.2 ± 9.3 | 21.4 ± 14.5* | 21.2 ± 10.7* | 20.5 ± 7.9* |
| AntiOx mixture | 26.8 ± 12.9 | 27.1 ± 8.8 | 27.4 ± 6.4 | 26.9 ± 13.3 | 25,7 ± 16.1 |
Table 2: Comparison of study parameters between the two treatment groups. In brackets: Values in healthy control subjects. *p<0.05 vs. baseline and vs. Antioxidant cocktail.
Serum level of cyclophilin-A and plasminogen activator inhibitor-1
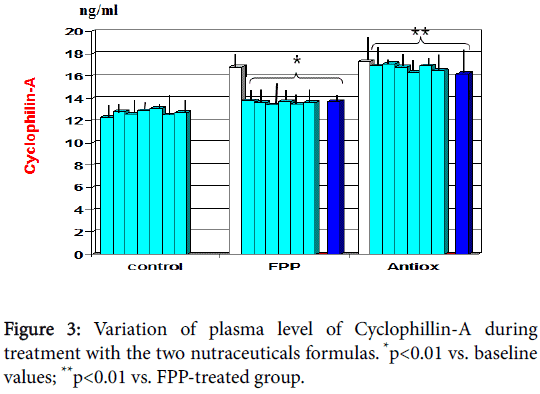
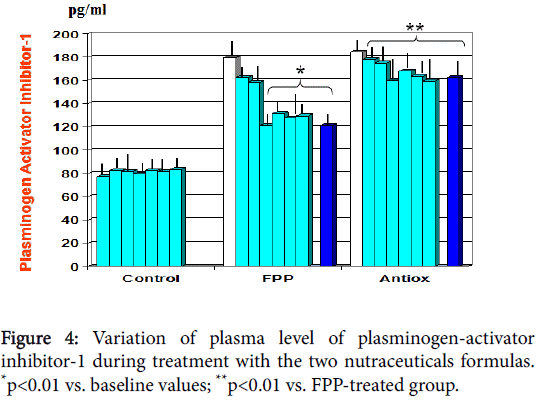
At baseline there appeared a significant correlation between plasminogen activator inhibitor-1 and uric acid (r: 0.62, p<0.05, not shown). As compared to baseline and to antioxidant cocktail control, FPP showed to significantly decrease serum levels of cyclophillin-A from 1st month observation (p<0.01, Figure 3) and plasminogen activator inhibitor-1 from 6 month observation (p<0.01, Figure 4). A significant correlation appeared between cyclophillin-A and oxidised- LDL (r: 0.69, p<0.05, not shown) but not with plasminogen activator inhibitor-1.
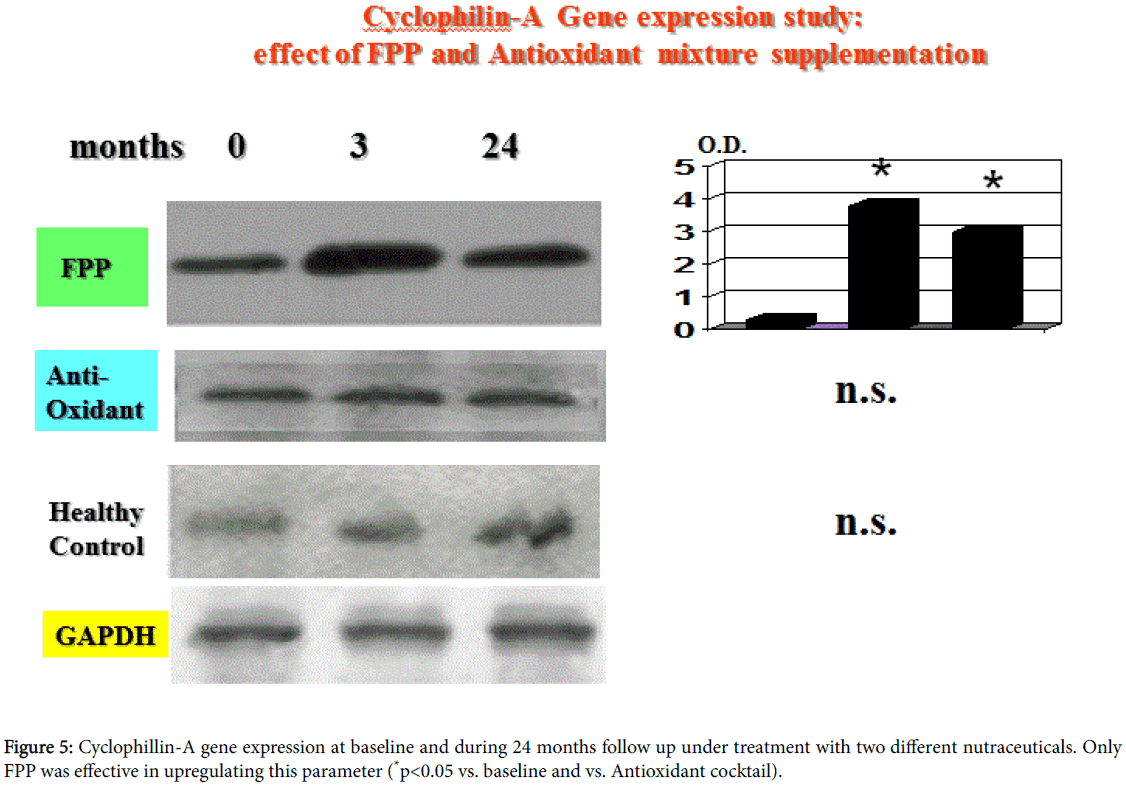
Cyclophilin-A gene expression study
At baseline observation, CyP-A gene expression was comparable with what observed in healthy controls (Figure 5). However, as compared to antioxidant cocktail-treated group, FPP enabled a significant upregulation at 3 and at 24 months (p<0.05, Figure 5).
Faecal heavy metal assessment
There was no correlation between this parameter, either when analysed as a single heavy metal or as a whole pooled value and any of the biochemical or gene expression values measured.
Irrespective of the group, all patients showed an overall abnormal faecal metals profile with the exception of nickel and arsenicum.
Unlike FPP or antioxidant control, the chelator-treated group showed a significantly greater faecal elimination of heavy metals (namely: mercury, cadmium, lead and beryllium (Table 3, p<0.05 vs. baseline and antioxidant combination). There was a not significant trend correlation between cumulative heavy metal values (either at baseline or after treatment) with oxidised-LDL or its antibody (data not shown).
| Months | Normal range | FPP | AntiOx mixture | Chelator | |
|---|---|---|---|---|---|
| Mercury mg/kg dry wt. |
21 24 |
<0.5 | (0.3±0.2) 0.4±0.3 |
(0.4±0.1) 0.4±0.2 |
(0.3±0.2) 1.1±0.3* |
| Cadmium mg/kg dry wt. |
21 24 |
<0.5 | (0.2±0.3) 0,5±0.14 |
(0.4 ±0.3) 0.5 ±0.12 |
(0.3±0.1) 1.4±0.31* |
| Lead mg/kg dry wt. |
21 24 |
<0.5 | (0.2±0.1) 0.2±0.6 |
(0.2 ±0.12) 0.3±0.09 |
(0.3 ±0.02) 1.1±0.41* |
| Arsenicum mg/kg dry wt. |
21 24 |
<0.3 | (0.1 ±0.04) 0.09±0.032 |
(0.1±0.08) 0.1±0.03 |
(0.3±0.04) 0.2±0.12 |
| Beryllium mg/kg dry wt. |
21 24 |
<0.009 | (0.004±0.003) 0.009±0.004 |
(0.007±0.004) 0.004±0.003 |
(0.009±0.004) 0.015±0.002* |
| Nickel mg/kg drywt. |
21 24 |
<8 | (5.8±0.8) 5.6±0.3 |
(6.4±1.2) 6.2±1.3 |
(6.1±0.14) 6.9 ±0.38 |
Table 3: Faecal heavy metal test: Effect of nutraceuticals with and without selected chelator. In brackets: baseline values corresponding to time 21 months and 24 month observation period of the trial. *p<0.05 vs. baseline and vs. antioxidant cocktail.
Discussion
Oxidative modification of LDLs may be a prerequisite for the rapid accumulation of LDLs within macrophages to form foam cells; indeed, oxidized LDL has been found in extracts from atherosclerotic lesions [3]. Oxidative modification of LDLs induces also the transformation of immunogenic epitopes in the LDL molecule, which lead to the formation of antibodies against oxidized LDLs in the serum [4]. Nevertheless, the clinical importance of these autoantibodies is still under discussion. For example, in patients with diabetes, no association has been found between anti-oxidized-LDL antibodies and microvascular complications [11], nor has an association been found with the levels of cholesterol in patients with heterozygous hypercholesterolemia [32] or with the intensity of serum oxidizability [33]. Others have reported an inverse relation between levels of cholesterol and anti-oxidized-LDL antibodies in the general population [34] and this seems to agree with the finding of an inverse link between IgM autoantibodies to oxidized LDL and carotid artery atherosclerosis [35]. The preliminary data of our study confirms that FPP unlike the control antioxidant, specifically decreased oxidized- LDL and, on the long run, also enhanced its antibodies concentration, although not modifying the lipid profile. Unlike what reported by Balada et al. [36], we did not notice any significant gender difference as for the levels of anti-oxidized LDL antibodies. While brain cholesterol metabolism is separated from the systemic circulation, oxidised cholesterol moieties may damage the functional integrity of bloodbrain barrier with potentially detrimental effects on neuronal population [37]. The same group has also shown on an experimental model that oxidised-LDL lipids and 27-OH-cholesterol may trigger Aβ production by GSH depletion and β-secretase-1 activation in neuronal cells [38]. It is noteworthy mentioning that a recent experimental study connects a fat-based/high calorie diet with metabolic syndrome, oxidative stress and significant hippocampal and temporal area degeneration together with memory dysfunction [39]. This evident histopathological finding is also in agreement with one of the current clinical hypothesis [40].
Moreover, only FPP decreased cyclophilin-A plasma level and plasminogen activator-inhibitor (PAI-1) (p<0.01). During inflammatory processes and following oxidative stress, CyPA is released into the extracellular compartment by inflamed cells [41,42] as a tentative protection response but this factor ends up being a potent chemoattractant for human monocytes and neutrophils. Cyclophilin A has indeed been shown to be more strongly associated with oxidative stress and inflammation than C-reactive protein [43]. Interestingly, prior small open pilot in-house studies testing same cohort of patients with an omega-3-rich sturgeon-based supplement (Caviarlieri, Swiss cap) did not beneficially affect the lipid profile, oxidised lipid parameters or CyPA. On the contrary, this latter variable showed a worsening change either as plasma level or as gene expression, making it a not advisable choice in metabolic syndrome or diabetes (J. Cervi, manuscript in preparation). The brain is characterized by a low content of antioxidant systems and the damage caused by oxidative stress is one of the earliest pathophysiological events in the development of Alzheimer's disease where plasma oxidized-LDL level has been positively correlated with disease severity [44].
A further independent cardiovascular risk factor [45] as well coaggravating parameter in metabolic syndrome [46] is represented by PAI-1, the major regulator of fibrinolysis and a stress-related factor. As mentioned above, unlike the antioxidant cocktail and the recent negative study testing high dose tocotrienol [47], FPP supplementation brought about a significant normalization of altered values of this parameter.
On the other hand, a one-year consumption of a nutraceutical rich in resveratrol, one of the ingredient of our antioxidant cocktail, had shown to improve PAI-1, but CyPA and oxidised lipid markers were not tested [48]. A vascular component of neurodegenerative disease pathophysiology is credited by several epidemiological studies [49] and Oh et al. [50] has shown that patients with MCI and AD had significantly higher plasma PAI-1 levels, These appeared to correlate with cognitive function thus being a potentially early biomarker for AD. The upregulation of PAI-1 has also been suggested to explain insufficient BDNF neurotrophic support and increased neurodegeneration while suppressing PAI-1 expression/activity increases Aβ degradation [51]. To the vascular hypothesis is likely to cooperate also an increased CyPA which may affect endothelial cells and vascular smooth muscle cells [52,53]. Whatever the main mechanisms, it seems that by matching the metabolic profiling and AD, there might be three subtypes; an inflammatory one, a noninflammatory type and a zinc deficiency-associated type affecting younger population [54]. In a prior study using a sturgeon eggsderived compound of putative anti-inflammatory effect (Caviarlieri, Swiss cap, Switzerland) we had shown to increase circulating level of BDNF in healthy occupationally-stressed individuals [55]. However, internal and follow up data showed that this was a rather short-lived effect and disappointingly unaffecting PAI-1, OxLDL, Cyp-A or proBDNF. So, its initially suggested neuroprotective effect in vitro (but not at brain gene expression level in animals) had to be abandoned on clinical ground by our group.
On the other hand, the downregulating effect played by FPP on CyPA gene expression, but not from the antioxidant control, may be of relevance, when considering that it has become clear that a hyperglicemia and oxidative stress milieu may stimulate this molecule [56] which, on its turn, drives the ox-LDL-mediated differentiation and activation of monocytes to foam cells [57,58].
Heavy metals gut clearance was not affected by any of the nutraceuticals. However, the chelator, by itself remarkably increased fecal gut discharge of heavy metals but only in those patients with abnormal baseline values. The addition of a chelator to the control nutraceuticals didn’t prove to yield better results while the addition to FPP showed a trend increase of fecal discharge of cadmium (p<0.07 vs. oral chelator alone, n.s.).
The identification and application of novel biomarkers in nutritional and life-style interventional plans may foster healthy longevity and beneficially affect the occurrence of concomitant illnesses. Our preliminary data suggest that FPP might play a noteworthy role within a wider and comprehensive preventive medicine strategy plan for impending metabolic diseases which are potential co-factors of neurodegenerative disease. Environmental neurotoxins disrupt protein processing as shown in experimental studies [26-28] while effective heavy metals chelators remain a further additive avenue to possibly pursue in selected cases.
Conclusion
As overall platform, virtuous dietary regimen such as the nutritional model of Mediterranean diet still maintains its importance to counter fight the cellular events involved in the atherosclerosis and neurodegenerative process [59,60]. However, given these promising results, longer studies addressing the issue of specific long term evidence-based nutraceutical implementations are highly awaited. Lastly, most recent research work points out the crucial importance of mitochondrial dynamics in metabolic and neurodegenerative diseases [61]. Indeed, exposure of environmental toxin, high-calorie intake, glucolipotoxicity brings about dysfunctional mitochondria which perpetuate ROS spilling over. Interestingly, the work of Collard et al. [62] in diabetic rats has shown how FPP optimised mitochondrial energetics. Such “cellular energetic” hypothesis as one of the possible FPP mechanisms also crossing the blood-brain-barrier has been recently envisaged by Hayashi Y. (in: Mantello A, Catanzaro C, He F, Cuffari B, Bissi L, Milazzo M, Lorenzetti A. Marotta F. Novel Nutrigenomics Avenues in Nutraceuticals Use: The Current Status of Fermented Papaya Preparation–Ed. E. Aguilar, Bioactive compounds: At the frontier between nutrition and pharmacology, 2016; 94-119).
Acknowledgments
This study was supported by an unbiased grant from Osato Research Institute, Japan (91%) and with a minor grant (9%) rom San Babila Clinic, Milano.
References
- Marotta F, Celep GS, Cabeca A, Polimeni P (2012) Novel concepts on functional foods and nutrigenomics in healthy aging and chronic diseases: a review of fermented papaya preparation research progress. Functional Foods in Health and Disease 2: 120-136.
- Santiago LA, Osato JA, Hiramatsu M, Mori A (1992) Fermented papaya preparation quenched free radicals and inhibited lipids peroxidation in iron- induced epileptic focus in rats. Oxygen Rad 4: 405-408.
- Aruoma OI, Hayashi Y, Marotta F, Mantello P, Rachmilewitz E, et al.(2010) Applications and bioefficacy of the functional food supplement fermented papaya preparation. Toxicology 278: 6-16.
- Zhang J, Mori A, Chen Q, Zhao B (2006) Fermented papaya preparation attenuates β-amyloid precursor protein: β-amyloid-mediated copper neurotoxicity in β-amyloid precursor protein and β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Neuroscience 143: 63-72.
- Marotta F, Chui DH, Jain S, Koike K, Zhou L, et al. (2011) Effect of a fermented nutraceutical on thioredoxin level and TNF-alpha signaling in cirrhotic patients. J Biol Regul Homeost Agents 25: 37-45.
- Tomella C, Catanzaro R, Illuzzi N, Cabeca A, Celep G, et al. (2014) The hidden phenomenon of oxidative stress during treatment of subclinical-mild hypothyroidism: a protective nutraceutical intervention. Rejuvenation Res 17: 180-183.
- Marotta F, Weksler M, Naito Y, Yoshida C, Yoshioka M, et al. (2006) Nutraceutical supplementation: effect of a fermented papaya preparation on redox status and DNA damage in healthy elderly individuals and relationship with GSTM1 genotype: a randomized, placebo-controlled, cross-over study. Ann N Y Acad Sci 1067: 400-407.
- Marotta F, Pavasuthipaisit K, Yoshida C, Albergati F, Marandola P (2006) Relationship between aging and susceptibility of erythrocytes to oxidative damage: in view of nutraceutical interventions. Rejuvenation Res 9: 227-230.
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, et al. (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485: 512-516.
- Pasetto L, Pozzi S, Castelnovo M, Basso M, Estevez AG, et al. (2016) Targeting Extracellular Cyclophilin A Reduces Neuroinflammation and Extends Survival in a Mouse Model of Amyotrophic Lateral Sclerosis. J Neurosci 37: 1413-1427.
- Bolner A, Micciolo R, Ottavio Bosello O, Nordera G (2016) Effect of papaya supplementation on oxidative stress markers in Parkinson’s disease. Oxid Antioxid Med Sci 5: 49-55
- Barbagallo M, Marotta F, Dominguez LJ (2015) Oxidative stress in patients with Alzheimer's disease: effect of extracts of fermented papaya powder. Mediators Inflamm
- Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, et al. (2010) Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75: 764-770.
- Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, et al. (2010) Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology 75: 1982-1987.
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, et al. (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68: 51-57.
- Pistollato F, Cano SS, Elio I, Vergar MM, Giampieri F, Battino M. (2015) The Use of Neuroimaging to Assess Associations Among Diet, Nutrients, Metabolic Syndrome, and Alzheimer's Disease. J Alzheimers Dis48:303-318.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, et al. (2015) Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 114:999-1012.
- Li X, Song D, Leng SX (2015) Link between type 2 diabetes and Alzheimer's disease: from epidemiology to mechanism and treatment. Clin Interv Aging 10:549-560.
- Kim B, Feldman EL (2015) Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp Mol Med 47: e149.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment ofHigh BloodCholesterol in Adults (Adult Treatment Panel III), “Third report of the National Cholesterol Education Program (NCEP) (2002) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106: 3143-3421.
- Sun YH, Nfor ON, Huang JY, Liaw YP (2015) Association between dental amalgam fillings and Alzheimer's disease: a population-based cross-sectional study in Taiwan. Alzheimers Res Ther 7: 65-69.
- Hare DJ, Rembach A, Roberts BR (2016) The Emerging Role of Metalloproteomics in Alzheimer's Disease Research. Methods Mol Biol 1303: 379-389.
- Souza-Talarico JN, Marcourakis T, Barbosa F, Moraes Barros SB, Rivelli DP, et al. (2017) Association between heavy metal exposure and poor working memory and possible mediation effect of antioxidant defenses during aging. Sci Total Environ 575: 750-757.
- Maya S, Prakash T, Madhu KD, Goli D (2016) Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed Pharmacother 83: 746-754.
- Escudero-Lourdes C (2016) Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 53: 223-235.
- Malecki EA (2001) Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull 55: 225-228.
- Zhang Z, Miah M, Culbreth M, Aschner M (2016) Autophagy in Neurodegenerative Diseases and Metal Neurotoxicity. Neurochem Res 41: 409-422.
- Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48: 203-213.
- Ketelhuth DF, Tonini GC, Carvalho MD, Ramos RF, Boschcov P, et al. (2008) Autoantibody response to chromatographic fractions from oxidized LDL in unstable angina patients and healthy controls. Scand J Immunol 68: 456-462.
- Declerck PJ, Moreau H, Jespersen J (1993) Multicenter evaluation of commercially available methods for the immunological determination of plasminogen activator inhibitor-1 (PAI-1). Thromb Haemost 70: 858-863.
- Cornelis R, Fuentes-Arderiu X, Bruunshuus I, Templeton D (1997) International Union of Pure and Applied Chemistry and International Federation of Clinical Chemistry, Committee on Nomenclature. Properties and Units (C-NPU): properties and units in the clinical laboratory sciences. IX. Properties and units in trace elements (IFCC-IUPAC technical report 1997). Eur J Clin Chem Clin Biochem 35: 833-843.
- Delimaris I,Georgopoulos S, Kroupis C, Zachari A, Liberi M, et al. (2008) Serum oxidizability, total antioxidant status and albumin serum levels in patients with aneurysmal or arterial occlusive disease. Clin Biochem 41: 706-711.
- Craig WY, Poulin SE, Neveux LM, Palomaki GE, Dostal DA (1995) Anti-oxidized LDL antibodies and antiphospholipid antibodies in healthy subjects: relationship with lipoprotein- and oxidation-related analytes. J Autoimmun 8: 713-726.
- Tinahones FJ, Gomez-Zumaquero JM, Rojo-Martinez G, Cardona F, Esteva de Antonio IE, et al. (2002) Increased levels of anti-oxidized low-density lipoprotein antibodies are associated with reduced levels of cholesterol in the general population. Metabolism 51: 429-431.
- Karvonen J,Paivansalo M, Kesaniemi YA, Horkko S (2003) Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108: 2107-2112.
- Balada E, Ordi-Ros J, Matas L, Mauri M, Bujan S, et al. (2002) Atherosclerosis and anti-oxidized low density lipoprotein antibodies in an elderly population. Med Clin (Barc) 119: 161-165.
- Dias IH, Polidori MC, Griffiths HR (2014) Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem Soc Trans 42: 1001-1005.
- Dias IH, Mistry J, Fell S, Reis A, Spickett CM, et al. (2014) Oxidized LDL lipids increase β-amyloid production by SH-SY5Y cells through glutathione depletion and lipid raft formation. Free Radic Biol Med 75: 48-59.
- Treviño S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, et al. (2015) A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse 69: 421-433.
- van Dijk G, van Heijningen S, Reijne AC, Nyakas C, van der Zee EA, et al. Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front Neurosci 9: 173.
- Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, et al. (2000) Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res 87: 789-796.
- Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, et al. (2005) Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol 175: 517-522.
- Taguchi I, Abe S, Inoue T (2013) Cyclophilin A is a promising predictor of coronary artery disease. Circ J 77: 321-232.
- Zhao Z, Zhou H, Peng Y, Qiu CH, Sun QY, et al. (2014) Expression and significance of plasma 3-NT and ox-LDL in patients with Alzheimer's disease. Genet Mol Res 13: 8428-8435.
- Nikolopoulos GK, Bagos PG, Tsangaris I, Tsiara CG, Kopterides P, et al. (2014) The association between plasminogen activator inhibitor type 1 (PAI-1) levels, PAI-1 4G/5G polymorphism, and myocardial infarction: a Mendelian randomization meta-analysis. Clin Chem Lab Med 52: 937-950.
- Aburto-Mejía E, Santiago-Germán D, Martínez-Marino M, María Eugenia Galván-Plata, Almeida-Gutiérrez E, et al. (2017) Hypofibrinolytic State in Subjects with Type 2 Diabetes Mellitus Aggravated by the Metabolic Syndrome before Clinical Manifestations of Atherothrombotic Disease. Biomed Res Int 2017: 6519704.
- Che HL, Kanthimathi MS, Loganathan R, Yuen KH, Tan AT, et al. (2017) Acute effects of a single dose of tocotrienols on insulinemic and inflammatory responses in metabolic syndrome subjects after a high-fat challenge. Eur J Clin Nutr 71: 107-114.
- Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, et al. (2012) I One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease.Am J Cardiol 110: 356-363
- Safouris A, Psaltopoulou T, Sergentanis TN, Boutati E, Kapaki E, et al. (2015) Vascular risk factors and Alzheimer's disease pathogenesis: are conventional pharmacological approaches protective for cognitive decline progression? CNS Neurol Disord Drug Targets 14: 257-269.
- Oh J, Lee HJ, Song JH, Park SI, Kim H (2014) Plasminogen activator inhibitor-1 as an early potential diagnostic marker for Alzheimer's disease. Exp Gerontol 60: 87-91.
- Gerenu G, Martisova E, Ferrero H, Carracedo M, Rantamäki T, et al. (2017) Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer's neuropathology and cognitive deficits. Biochim Biophys Acta 1863: 991-1001.
- Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, et al. (2000) Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res 87: 789-796.
- Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC (2006) Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res 98: 811-817.
- Bredesen DE (2015) Metabolic profiling distinguishes three subtypes of Alzheimer's disease. Aging (Albany NY) 7: 595-600.
- Chui DH, Marcellino M, Marotta F, Sweed H, Solimene U, et al. (2014) A double-blind, rct testing beneficial modulation of BDNF in middle-aged, life style-stressed subjects: a clue to brain protection? J Clin Diagn Res 8: MC01-MC06.
- Tsai SF, Hsieh CC, Wu MJ, Chen CH, Lin TH, et al. (2016) Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin Chim Acta 463: 181-192.
- Xue Z, Yuan W, Li J, Zhou H, Xu L, et al. (2017) Cyclophilin A mediates the ox-LDL-induced activation and apoptosis of macrophages via autophagy. Int J Cardiol 230: 142-148.
- Ramachandran S, Vinitha A, Kartha CC (2016) Cyclophilin A enhances macrophage differentiation and lipid uptake in high glucose conditions: a cellular mechanism for accelerated macro vascular disease in diabetes mellitus. Cardiovasc Diabetol 15: 152-158.
- Casas R, Sacanella E, Estruch R (2014) The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets 14: 245-254.
- Claassen JA (2015) New cardiovascular targets to prevent late onset Alzheimer disease. Eur J Pharmacol 763: 131-134.
- Jha SK, Jha NK, Kumar D, Ambasta RK, Kumar P (2016) Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neurodegeneration. Biochim Biophys Acta pii: S0925-S4439.
- Collard E, Roy S (2010) Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid Redox Signal 13: 599-606.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 5561
- [From(publication date):
May-2017 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 4591
- PDF downloads : 970