A 1-year Comparison of Generic Tacrolimus (Tacni�?®) and Prograf�?® in Renal Transplant Patients: A Retrospective Matched Group Analysis
Received: 15-Feb-2017 / Accepted Date: 15-May-2017 / Published Date: 19-May-2017 DOI: 10.4172/2475-7640.1000113
Abstract
Introduction: The 1-year-outcome of adult patients treated with Tacni® or Prograf® following de novo renal transplantation, including patient survival, graft loss, Biopsy Proven Acute Rejection (BPAR) and serious adverse events were compared. 186 patients who underwent their first renal transplantation between 2011 and 2014 were included in a retrospective matched group analysis (91 patients treated with Tacni® which were compared with a matched control group (n=95).
Material and Methods: This was a retrospective study of two different time cohorts of patients who underwent kidney transplantation at Sahlgrenska University Hospital. Data was obtained from patient charts and from the local quality registry. Patients from the two groups were matched in a group matching fashion. The matching was based upon the patient’s age and gender, age of the donor, living/deceased donor and Cold Ischemia Time (CIT).
Results: The two groups were well matched for living donor transplants; gender; donor and recipient age. The combined endpoint of freedom from BPAR, graft loss and death 12 months post-transplantation was 85% in the P-group and 86% in the T-group (p=0.90). There was one death in the T-group, which was not related to the drug (p=1.00). Graft survival at 12 months was similar, 99% in both groups (p=0.97). The BPAR at 12 months was 12% (T) and 14% (P), respectively (p=0.72). The measured GFR at 12 months was also similar, 54.5 and 56.0 ml/min/1.73 m2 (p=0.59). There were no significant differences in Tacrolimus levels.
Conclusion: One year after transplantation, generic Tacrolimus is no different to the original version. Both drugs show good efficacy, the safety is comparable and the drug concentrations do not differ.
Keywords: Renal transplantation; Immunosuppression; Tacrolimus; Generic
15385Introduction
Corticosteroids and Calcineurin Inhibitors (CNIs), such as Tacrolimus and ciclosporin, are the main molecules used in immunosuppressive renal transplantation protocols throughout the world. Following the expiration of the patent protection of Tacrolimus in 2009, several generic versions of the drug have entered the market. However, with the increasing use of these generic immunosuppressants, well designed studies comparing the original Tacrolimus (Prograf®; Astellas Pharma Inc., Tokyo, Japan) with generic Tacrolimus in order to ensure the long-term safety of the drug are required [1].
The first bioequivalence of a generic Tacrolimus was demonstrated in the US in 20 healthy volunteers in 2009 and the drug was thereafter approved by the United States Federal Drug Administration. However, due to the significant differences in drug metabolism between healthy volunteers and transplant patients, many suggest that meeting the criteria of bioequivalence in healthy individuals is insufficient to ensure the safety and efficacy of generic substitutions of Narrow Therapeutic Index (NTI) drugs such as Tacrolimus [2,3]. In addition, a recent study showed that Tacni® (Teva UK) did not meet the bioequivalence criteria in elderly transplant patients and that the use of Tacni® resulted in a significantly higher systemic drug exposure. In the long run, this may put the patients at higher risk of Calcineurin Inhibitor-Related Toxicity (CIRT) and impaired long-term outcomes [2].
The trough concentrations and dose requirements of generic Tacrolimus are shown to be comparable to brand-name Tacrolimus by several studies performed in the past few years [4-7]. Nevertheless, dose titration is often required after the switch from the reference drug to generic Tacrolimus and close therapeutic drug monitoring is needed in order to achieve a satisfactory balance between maximizing efficacy and minimizing dose-related toxicity. Due to the cost-saving benefits and the overall evidence from several studies suggesting that generic Tacrolimus appears safe, switching from brand-name Tacrolimus is widely encouraged. However, the follow-up period in these studies was brief (maximum nine months) and there was no observation of the long-term outcome of patients [4-7]. In addition, there are only two studies to date that have studied patients who were treated with generic Tacrolimus on the day of discharge after renal transplantation, as opposed to being switched from reference Tacrolimus [2,3]. Considering this, together with the concerns about bioequivalence in elderly patients, data showing comparable clinical effectiveness of generic and brand-name Tacrolimus are needed [1].
The primary aim of the study was to investigate potential differences regarding the overall safety and efficacy of generic versus original Tacrolimus during the first year after transplantation.
Materials and Methods
Study design
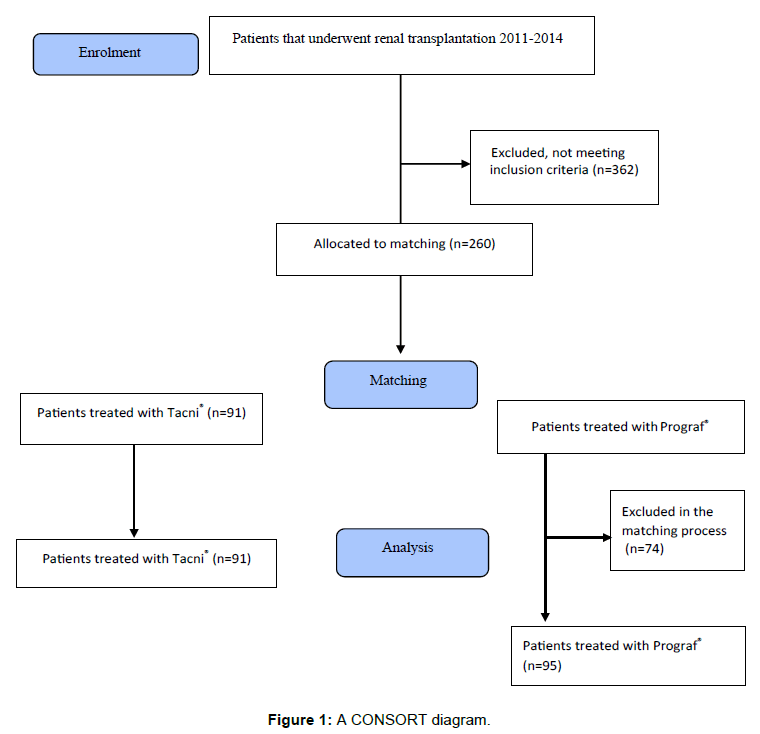
This was a retrospective, non-powered study of two different time cohorts of patients who underwent kidney transplantation at Sahlgrenska University Hospital (SU). Data was obtained from patient charts and from TIGER, the local quality registry. Sample size was based on utilizing all renal transplant patients on Tacni®, comparing them with a matched group of patients treated with Prograf® on day of discharge. Patients from the two groups were matched in a group matching fashion. The matching was based upon the patient’s age and gender, age of the donor, living/deceased donor and Cold Ischemia Time (CIT). The closest matches were applied to living vs. deceased donors. Second closest matching was applied to age and CIT, which were kept within two years and three hours, respectively.
The primary objective of the study was to investigate the combined endpoint of freedom from Biopsy Proven Acute Rejection (BPAR), graft loss and death 1 year after transplantation in a population treated with generic Tacrolimus compared with a population, treated with Prograf®.
Secondary objectives were to compare the following parameters after 1 year:
• Graft survival.
• BPAR at 6 and 12 months.
• Calculated glomerular filtration rate (eGFR) according to the MDRD equation and.
• Measured GFR (if available).
• Serious adverse events at 6 and 12 months.
• Tacrolimus levels at post-operative day 4, 7, 14 and 21.
Patients
Adult patients (18+ years) receiving a first single kidney transplant from a deceased or living donor who were treated with Tacni® or Prograf® on day of discharge were enrolled. Exclusion criteria included multi-organ transplants or previous renal or non-renal transplants, patients under 18 years of age, as well as patients transplanted before the year of 2011. A total of 622 patients were screened using the local registry at Transplantationszentrum (TIGER) at SU. 91 patients on Tacni® met the eligibility criteria and were enrolled in the study. 169 Prograf® patients were identified; from this group 95 were selected from the matching process (Figure 1). In total 186 patients could be included in the study.
Immunosuppression
Standard immunosuppressive regimen consisted of Tacrolimus, mycophenolate mofetil, and steroids. Induction therapy was given with two doses of Basiliximab. According to the center transplant protocol, Tacrolimus was initiated the day after transplantation at a dose of 0.05 mg/kg twice daily, aiming at a target level of 5-9 ng/ml during the first 3 months. MMF was initiated in a dose of 1 g twice daily. Prednisolon was initiated at a dose of 50 mg twice daily with a weaning to 10 mg daily at 1 month and 5 mg daily at three months.
Statistical analysis
The matching process was conducted utilizing population matching by minimizing t-values based on living/deceased donor, donor age, recipient age and CIT. Statistical analysis was carried out to compare preoperative patient characteristics, perioperative primary outcomes, and complications using the Chi-square test, Fisher’s Exact test (for dichotomous variables) and the Mann-Whitney U-test (for continuous variables). The study was approved by the Regional Ethics Committee in Gothenburg (Dnr: 475-15).
Results
The primary aim of the study was to investigate potential differences regarding the overall safety and efficacy of generic versus original Tacrolimus; more specifically to compare the 1-year-outcome of adult patients treated with Tacni® or Prograf® following de novo renal transplantation. The baseline characteristics of the study population are shown in Table 1.
| Variable | Prograf® (n=95) |
Tacni® (n=91) |
p-value |
|---|---|---|---|
| Age | 53.8 (22.4- 74.5) | 52.3(21.1-73.0) | 0.84 |
| Sex | - | - | 0.48 |
| Male | 54 (56.8%) | 46 (50.5%) | - |
| Female | 41 (43.2%) | 45 (49.5%) | - |
| Race (for eGFR): | -p | - | 1.00 |
| Black | 0 (0.0%) | 0 (0.0%) | - |
| Other | 95 (100.0%) | 91 (100.0%) | - |
| Donor living or deceased | - | - | 1.00 |
| Living | 37 (38.9%) | 35 (38.5%) | - |
| Deceased | 58 (61.1%) | 56 (61.5%) | - |
| Donor age (years): | 55.0 (11.0-74.0) | 53.0 (7.0-80.0) | 0.50 |
Table 1: Demographics and baseline characteristics. For categorical variables n (%) is presented and for continuous variables median (min-max). Chi-square test and Fisher's Exact test was applied for dichotomous variables and Mann-Whitney U-test for continuous variables.
The study groups were well matched for living/deceased donor, donor age, recipient age and CIT. The combined endpoint of freedom from patient death, graft loss or rejection which was the primary endpoint of the study showed no difference between the two groups.
85.3% of Prograf® patients and 85.7% of Tacni® (p=0.90) patients were free of complications within one year of transplantation, including death, graft loss or rejection (Table 2).
| Variable | Prograf® | Tacni® | p-value |
|---|---|---|---|
| (n=95) | (n=91) | ||
| Age | 53.8 (22.4- 74.5) | 52.3(21.1-73.0) | 0.84 |
| Sex | - | - | 0.48 |
| Male | 54 (56.8%) | 46 (50.5%) | |
| - | |||
| Female | 41 (43.2%) | 45 (49.5%) | - |
| Race (for eGFR) | - | - | 1 |
| Black | 0 (0.0%) | 0 (0.0%) | |
| - | |||
| Other | 95 (100.0%) | 91 (100.0%) | - |
| Donor living or deceased | - | - | 1 |
| Living | 37 (38.9%) | 35 (38.5%) | - |
| Deceased | 58 (61.1%) | 56 (61.5%) | - |
| Donor age (years): | 55.0 (11.0-74.0) | 53.0 (7.0-80.0) | 0.5 |
Table 2: Comparison of renal function and survival. For categorical variables n (%) is presented and for continuous variables median (min-max) Chi-square test and Fisher's Exact test was applied for dichotomous variables and Mann-Whitney U-test for continuous variables.
Out of the 186 subjects that were included in the study, only one death was recorded due to postoperative bleeding, in a patient treated with Tacni® (Table 2). The cause of death was not related to the choice of immunosuppression. One subject in each group experienced graft loss (Table 2).
BPAR was recorded in 24 (12.3%) of the 186 subjects. 13 (13.7%) and 11 (12.1%) patients experienced BPAR in the Prograf® and Tacni® group, respectively (p=0.72) (Table 2).
The measured GFR (mGFR) at 12 months was available in 79 patients (83%) in the Prograf® group and 75 patients (82%) in the Tacni® group (Table 2). mGFR was measured using iohexol-clearance. The median value of mGFR was 56.0 (22.0-103.0) and 56.0 (7.0- 91.0) mL/min/1.73 m2 for Prograf® and Tacni®, respectively (p=0.59). eGFR at 12 months was calculated according to the MDRD-formula. S-creatinine levels at 12 months were available in 90 Prograf® and 89 Tacni® patients. The median value of eGFR was estimated to 59.4 (21.5- 127.5) mL/min/1.73 m2 for Prograf® and 59.7 (4.8-122.8) mL/min/1.73 m2 for Tacni® (p=0.45), so no significant difference in renal function was observed between the groups, according to both mGFR and eGFR.
Serious adverse events were defined according to European Medicines Agency guidance. The events were grouped into 13 categories (Table 3). As demonstrated in the table, there was no difference in the number of SAE between the groups.
| Variable | Prograf® (n=95) |
Tacni® (n=91) |
|---|---|---|
| At 12 months | At 12 months | |
| Subjects with SAE, n (%) | 41 (43.2%) | 43 (47.3%) |
| Total number of SAE | 85 | 75 |
| Rejection | 14 | 14 |
| Kidney disease | 3 | 6 |
| UTI | 7 | 4 |
| Other infection | 16 | 9 |
| Bleeding | 2 | 4 |
| Lymphocele | 6 | 0 |
| Tumor | 3 | 4 |
| Cardiovascular disease | 5 | 2 |
| Other | 8 | 12 |
| Neutropenia | 2 | 0 |
| Sepsis | 13 | 11 |
| Uretary complications | 4 | 7 |
| Diabetes | 0 | 2 |
Table 3: Serious adverse events: Any recorded event requiring or prolonging hospital stay post-transplantation.
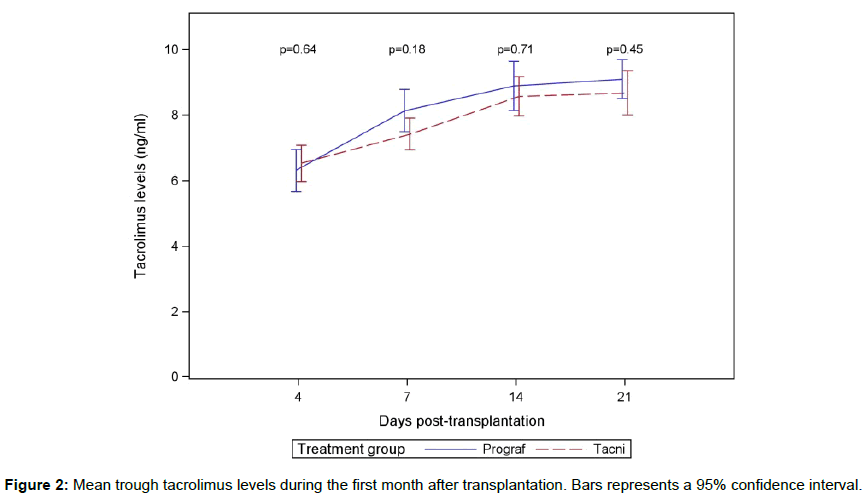
Tacrolimus levels increased over time and stabilized between 14 and 21 days at a level between 8 and 9 ng/ml (Figure 2). There was no significant difference in mean value and standard deviation between the two groups. The accumulated doses of Tacrolimus were not measured in the study.
Discussion and Conclusion
The use of generic immunosuppressive drugs in transplantation is a controversial topic. There is a consensus among transplant societies that clinical data is lacking and that caution should be exercised [8]. The reluctance to use generic drugs such as Tacni® in organ transplantation is partly related to the fact that most are NTI drugs (sometimes called “critical dose drugs”), and that either low dosing or overdosing could have serious consequences for both patients and society [9]. Another controversy is whether a demonstration of bioequivalence by a single fixed dose study in healthy adults can offer a sufficient guarantee of therapeutic equivalence in solid organ transplant patients [10].
A number of groups have shown acceptable short-term outcomes with generic Tacrolimus formulations in non-randomized clinical studies in de novo kidney transplant recipients. Most of these were conversion studies and compared Prograf® to the generic formulations Tacrobell® and Pan Graf® [4,11,12].
In one of the two published de novo studies Adoport® was compared with Prograf® [13]. There were no differences in 6-month outcomes regarding clinical results, histology in protocol biopsies, and formation of donor specific antibodies. In the other de novo study [3], focusing on pharmacokinetics in 117 patients, efficacy and safety data were comparable over the 9-month study period.
There is only one study comparing Tacni to Prograf® [3], which noticed a higher drug exposure with Tacni® in elderly patients. To the best of our knowledge, our current study is the largest to evaluate de novo use of generic Tacrolimus and the first study comparing the 1-year outcomes of Tacni®. The aim of the study was to investigate potential differences regarding the overall safety and efficacy. When designing the study, we wanted to create as large a sample size as possible to increase the statistical power. The limiting factor was that Tacni® has only been part of the standard immunosuppression regimen at our institution for three years and therefore the number of kidney transplant patients on Tacni® was restricted to approximately 100 patients. Since Prograf® was the only Tacrolimus drug on the market for many years; we had a larger pool of patients to choose from when selecting our control group.
For this reason, we closely matched the control group with the Tacni® group based on gender, living/deceased donor, donor age, recipient age and CIT. By matching by group rather than in a 1:1 ratio we were able to make the groups more closely correlated to one another, thus eliminating the most evident confounders and increased the strength of the result. Except for the Tacrolimus brand, the immunosuppressive protocol was identical in the two groups. Even if we cannot exclude any potential differences in bioavailability in a subgroup of patients, we did not see any evidence of this and overall the one-year efficacy and safety of renal transplantation with Tacni® was not different compared to Prograf®.
Interchangeability between generic Tacrolimus and Prograf® is an important issue in solid organ transplantation for health economic reasons. The switch in protocol at our institution from Prograf® to Tacni® in 2013 resulted in significant savings, as Tacni® was approximately 40% cheaper than reference Tacrolimus in Sweden. In general, generic medicines are typically 20 to 90% cheaper than originator equivalents. For example, in 2010 alone, the use of generics in the American health system saved $158 billion (an average of $3 billion every week) and a study by the Generic Pharmaceutical Association (GPhA) showed that prescribing of generic products saved the US economy $931 billion between 2001 and 2010 [14].
Compared to most other studies, this is one of the few which analyses data of patients who were treated with generic Tacrolimus on the day of discharge following renal transplantation, as opposed to being switched from reference Tacrolimus [2,3]. More data supporting the use of generic Tacrolimus will be generated from transplant registries which can provide evidence about long-term graft survival in larger patient cohorts.
The absence of evidence of differences is not equal to evidence of no differences [8], but still the results obtained in this study supports that the use of generic Tacrolimus is a safe method to improve health economy in transplantation.
In conclusion, in this study, one-year post-transplantation, immunosuppression with generic Tacrolimus (Tacni®) is not different from medication with the original version. Both drugs show good efficacy, the safety is comparable and the drug concentrations do not differ.
References
- Molnar A, Fergusson D, Tsampelieros A, Bennett A, Fergusson N, et al. (2015) Generic immunosuppression in solid organ transplantation: systematic review and metaanalysis. BMJ 350: 3163.
- Robertsen I, Asberg A, Ingero AO, Vethe NT, Bremer S, et al. (2015) Use of generic tacrolimus in elderly renal transplant recipients: precaution is needed. Transplantation 99: 528-532.
- Min SI, Ha J, Kim YS, Ahn SH, Park T, et al. (2013) Therapeutic equivalence and pharmacokinetics of generic tacrolimus formulation in de novo kidney transplantations. Nephrol Dial Transplant 28: 3110.
- McDevitt-Potter LM, Sadaka B, Tichy EM, Rogers CC, Gabardi S (2011) A multicentre experience with generic tacrolimus conversion. Transplantation 92: 653.
- Rosenborg S, Nordström A, Almquist T, Wennberg L, Bárány P (2014) Systematic conversion to generic tacrolimus in stable kidney transplant patients. Clin Kidney J 7: 151-155.
- Spence MM, Nguyen LM, Hui RL, Chan J (2012) Evaluation of clinical and safety outcomes associated with conversion from brand?name to generic Tacrolimus in transplant recipients enrolled in an integrated health care system. Pharmacotherapy: Pharmacotherapy 32: 981-987.
- Momper JD, Ridenour TA, Schonder KS, Shapiro R, Humar A, et al. (2011) The impact of conversion from prograf to generic tacrolimus in liver and kidney transplant recipients with stable graft function. Am J Transplant 11: 1861-1867.
- Gelder TV (2011) European society for organ transplantation advisory committee recommendations on generic substitution of immunosuppressive drugs. Transpl Int 24: 1135-1141.
- Allard J, Fortin MC (2014) Is it ethical to prescribe generic immunosuppressive drugs to renaltransplant patients? Can J Kidney Health Dis 1: 23.
- Harrison JJ, Schiff JR, Coursol CJ, Daley CJ, Dipchand AI, et al. (2012) Generic immunosuppression in solid organ transplantation: a Canadian perspective. Transplantation 93: 657-665.
- Kim SJ, Huh KH, Han DJ, Moon IS, Kim SJ, et al. (2009) A 6-month, multicenter, single-arm pilot study to evaluate the efficacy and safety of generic tacrolimus (TacroBell) after primary renal transplantation. Transplant Proc 41: 1671-1674.
- Guleria S, Kamboj M, Chatterjee A, Sharma M, Awasthy V, et al. (2008) Generic tacrolimus (Pan Graf) in renal transplantation: an experience of 155 recipients in India. Transplant Proc 40: 2237-2239.
- Melilli E, Crespo E, Sandoval D, Manonelles A, Sala N, et al. (2015) De novo use of a generic formulation of tacrolimus versus reference tacrolimus in kidney transplantation: evaluation of the clinical results, histology in protocol biopsies, and immunological monitoring. Transpl Int 28: 1283-1290.
- Dunne S, Shannon B, Dunne C, Cullen W (2013) A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol 14: 1.
Citation: Lindnér C, Lindnér P (2017) A 1-year Comparison of Generic Tacrolimus (Tacni®) and Prograf® in Renal Transplant Patients: A Retrospective Matched Group Analysis. J Clin Exp Transplant 2: 113. DOI: 10.4172/2475-7640.1000113
Copyright: © 2017 Lindnér C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6966
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 6153
- PDF downloads: 813