Synchronous HPV-Positive Squamous Cell Carcinoma of the Bilateral Base of the Tongue
Received: 12-Jan-2014 / Accepted Date: 18-Mar-2014 / Published Date: 25-Mar-2014 DOI: 10.4172/2161-119X.1000164
Abstract
Although there have been several reports of synchronous Human Papillomavirus (HPV)-associated tonsillar carcinoma, no reports have shown synchronous HPV-associated Base of The Tongue (BOT) carcinoma. Here we discuss a case of synchronous HPV-associated BOT carcinoma and review the literature concerning HPV-associated second primary tumors. We report the case of a 59-year-old man with synchronous HPV-positive Squamous Cell Carcinoma (SCC) of the bilateral BOT. Both BOT SCCs were positive for HPV16. The patient received concomitant cisplatin radiotherapy, and remains disease-free at 18 months post-treatment.
Keywords: HPV, Base of tongue, Synchronous, Squamous cell carcinoma
246202Introduction
Patients with Head and Neck Squamous Cell Carcinoma (HNSCC) are at increased risk for Second Primary Tumors (SPTs) compared with the general population [1]. These SPTs typically develop in the aerodigestive tract and contribute significantly to the shortened survival of patients treated for HNSCC [2]. SPTs are categorized as either synchronous or metachronous SPTs, according to their temporal relationship to the index tumor. Synchronous SPTs are identified as occurring within six months of the index tumor, whereas metachronous SPTs are diagnosed more than six months after diagnosis of the index tumor. Patients with a synchronous SPT have a worse prognosis than those presenting with a metachronous SPT [3].
In recent years, the incidence of human papilloma virus (HPV)- related Oropharyngeal Squamous Cell Carcinomas (OPSCCs) has been rising in Western Europe and the United States, while the prevalence of smoking- and alcohol-induced carcinoma has been declining [4]. HPVassociated tumors predominantly arise in the tonsillar region or BOT. Patients with HPV-related OPSCCs have better survival than those with HPV-negative tumors [5]. We have previously reported that Japanese patients with HPV-associated OPSCCs have markedly superior survival rates compared to those with HPV-negative OPSCCs [6].
Recent studies have shown that patients with HPV-associated HNSSCs are less likely to develop SPTs [5]. However, these patients may be at an elevated risk of developing other HPV-associated carcinomas. Although there have been a number of recent reports of synchronous HPV-associated tonsillar carcinomas, there have been no reports of synchronous HPV-associated BOT carcinoma. Here we report the case of a 59-year-old man with synchronous HPV-positive SCCs of the bilateral BOT [7-9].
Case Report
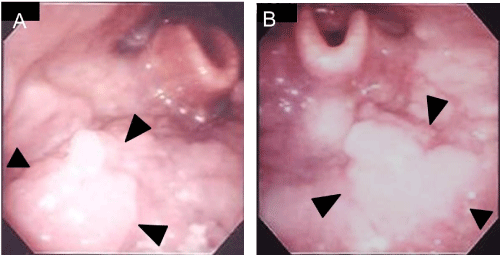
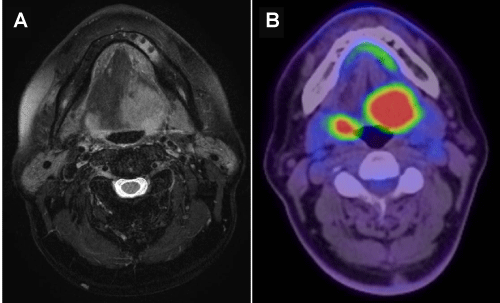
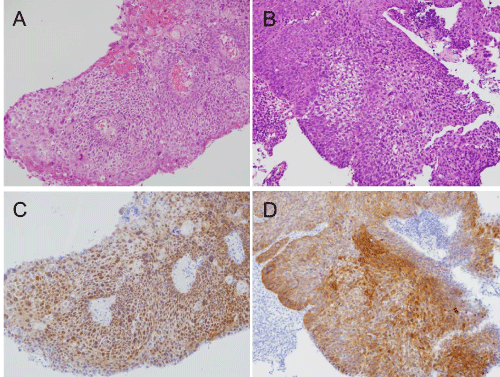
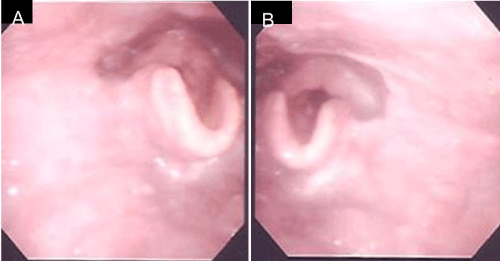
A 59-year–old man presented with pain around the left side of the tongue of two months’ duration. He had a history of smoking over one pack of cigarettes per day for 24 years, and consuming approximately 350 ml of beer per day for 40 years. Fiberoptic laryngoscopy showed tumors on both the left and right sides of the BOT (Figure 1). Initial MR imaging showed a 38-mm mass on the left BOT and a 13-mm mass on the right BOT (Figure 2A). Positron emission tomography (PET)/CT scanning revealed 18F-Fluorodeoxyglucose (FDG)-avid masses on both sides of the BOT (Figure 2B). There was no evidence of lymph node metastasis or distant metastasis on the PET/CT scans. Biopsies of both BOT tumors were taken and pathologic examination showed invasive SCCs of the left and right BOT. The photographed area on the right BOT showed a moderately differentiated squamous cell carcinoma (Figure 3A). The photographed area on the left BOT indicated a poorly differentiated squamous cell carcinoma (Figure 3B). Both BOT SCCs (nuclear and cytoplasmic) were positive for P16 immunohistochemical staining as well as for HPV16 by multiplex-PCR, which can detect 16 HPV genotypes (types 6, 11, 16, 18, 30, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) with high sensitivity and a high degree of reproducibility [6] (Figure 3C and 3D). The left tumor was staged as T2N0M0, and the right tumor was staged as T1N0M0. After complete staging, the patient received concomitant cisplatin radiotherapy at a total dose of 68 Gy. Post-treatment, neither tumor could be detected by fiberoptic laryngoscopy (Figure 4). Follow-up PET/CT studies obtained 2 months after the completion of radiation revealed a complete metabolic response. At 18 months after the completion of radiotherapy, no evidence of recurrence or metastasis was observed.
Figure 2: Axial T2-weighted MR images before treatment.
A) 38-mm mass on the left BOT and a 13-mm mass on the right BOT are shown. B) Positron emission tomography (PET)/CT scans before treatment. Bilateral BOT tumors were demonstrated and PET/CT scans demonstrated hypermetabolic activity in the bilateral BOT.
Figure 3: Histopathologic findings of the biopsy.
Hematoxylin and eosin-stained sections of the right (A) and left (B) BOT tumors p16 immunostaining of the right (C) and left (D) BOT tumors. High levels of p16 expression were observed in the nucleus and cytoplasm of both the right and left BOT tumors.
Discussion
We herein report the case of a 59-year-old man with synchronous SCCs of the bilateral BOT. Several reports have described patients diagnosed with HPV-HNSCCs who subsequently developed SPTs of the head and neck; however, the SPTs in these reports developed in the tonsils or nasopharynx [7-9]. Our review of the literature suggests that this is the first report of synchronous HPV-positive SCCs of the bilateral BOT.
HPV-positive tumors are epidemiologically, clinically, and biologically different from HPV-negative tumors. Smoking and alcohol use are risk factors for HPV-negative SCCs. These agents are thought to contribute to changes throughout the epithelium, and their continued use is associated with an increased risk of SPTs. SPTs pose an obstacle to long-term survival among patients with HNSCCs [10]. On the other hand, recent reports have shown that HPV-positive OPSCC patients were less likely to have SPTs [5]. Huang et al. [11] reported that a decrease in SPTs, including prior tumors, synchronous lesions, and metachronous SPTs (11% vs. 20%, 1% vs. 9%, and 6% vs. 13%, respectively). Morris et al. [1] reported that, although oropharynx index carcinoma carried the second highest excess burden of SPT of any head and neck sub-site prior to the 1990s, it currently carries the lowest excess burden of SPT. This is due to the lower exposure to tobacco and younger age in HPV-positive HNSCC patients.
Patients with HPV-associated HNSSCs are less likely to develop SPTs. However, these patients may be at an elevated risk of developing other HPV-associated carcinomas. It has been shown that patients diagnosed with OPSCCs are more likely to develop cervical carcinoma than is the general population [12]. It has also reported that patients with a history of cervical carcinoma are at increased risk of a subsequent SPT in the head and neck region [13]. The incidence of second primary HPV-associated carcinoma is currently unknown. Joseph et al. [8] reported an incidence of 4% for SPTs in the contralateral tonsil of patients presenting with an index HPV-associated tonsillar carcinoma.
The molecular mechanism underlying the development of SPTs in the context of HPV-HNSCC remains unknown. Joseph et al. [8] reported that in four patients with bilateral HPV-related tonsillar carcinomas, the HPV DNA sequences derived from the index tumor and corresponding SPT were 100% identical. They also speculated that HPV-related tumor multi-focality can be attributed either to independent inoculation events by the same virus, or by migration of HPV-related cells from a single inoculation site to other regions of Waldeyer’s ring. In our case, as HPV16 was detected in both BOT tumor sites, the second mechanism appears more likely.
Recent meta-analysis suggests that the prevalence of HPV in cases of OPSCC was 72.2% between 2005 and 2009 in North America and Europe [14]. In Japan, we previously reported that the prevalence of HPV in cases of OPSCC was 32.4% between 1998 and 2009. The rate of tobacco consumption is also decreasing in Japan, and it is likely that not only the prevalence of HPV-associated OPSCC, but also of synchronous HPV-associated HNSCC may be increasing.
Conclusion
We have presented the case of a 59-year-old man with synchronous HPV-positive SCCs of the bilateral BOT. Along with an increased incidence of HPV-related HNSCC, synchronous HPV-associated HNSCC may also be increasing.
Acknowledgements
Study performed at Hokkaido University Hospital, Sapporo, Japan.
Financial support: Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Morris LGT, Sikora AG, Patel SG, Hayes RB, Ganly I (2010) Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 29: 739-746.
- Bosetti C, Scelo G, Chuang SC, Tonita JM, Tamaro S, et al. (2011) High constant incidence rates of second primary cancers of the head and neck: a pooled analysis of 13 cancer registries. Int J Cancer 129: 173-179.
- Rennemo E, Zätterström U, Boysen M (2008) Impact of second primary tumors on survival in head and neck cancer: an analysis of 2,063 cases. Laryngoscope 118: 1350-1356.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- Mizumachi T, Kano S, Sakashita T, Hatakeyama H, Suzuki S, et al. (2013) Improved survival of Japanese patients with human papillomavirus-positive oropharyngeal squamous cell carcinoma. Int J ClinOncol 18: 824-828.
- McGovern SL, Williams MD, Weber RS, Sabichi A, Chambers MS, et al. (2010) Three synchronous HPV-associated squamous cell carcinomas of Waldeyer's ring: case report and comparison with Slaughter's model of field cancerization. Head Neck 32: 1118-1124.
- Joseph AW, Ogawa T, Bishop JA, Lyford- Pike S, Chang X, et al. (2013) Molecular etiology of second primary tumors in contralateral tonsils of human papillomavirus-associated index tonsillar carcinomas. Oral Oncol 49: 244-248.
- Moualed D, Qayyum A, Price T, Sharma A, Mahendran S (2012) Bilateral synchronous tonsillar carcinoma: a case series and review of the literature. Eur Arch Otorhinolaryngol 269: 255-259.
- Vikram B (1984) Changing patterns of failure in advanced head and neck cancer. Arch Otolaryngol 110: 564-565.
- Huang SH, Perez-Ordonez B, Liu FF, Waldron J, Ringash J, et al. (2012) Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J RadiatOncolBiolPhys 82: 276-283.
- Biron VL, Côté DW, Seikaly H (2011) Oropharyngeal squamous cell carcinoma and human papillomavirus-associated cancers in women: epidemiologic evaluation of association. J Otolaryngol Head Neck Surg 40 Suppl 1: S65-69.
- Rose Ragin CC, Taioli E (2008) Second primary head and neck tumor risk in patients with cervical cancer--SEER data analysis. Head Neck 30: 58-66.
- Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, et al. (2013) Prevalence of human papillomavirus in oropharyngeal and non-oropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 35: 747-755.
Citation: Mizumachi T, Sakashita T, Hishimura Y, Kakizaki T, Homma A, et al. (2014) Synchronous HPV-Positive Squamous Cell Carcinoma of the Bilateral Base of the Tongue. Otolaryngology 4:164. DOI: 10.4172/2161-119X.1000164
Copyright: © 2014 Mizumachi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 19541
- [From(publication date): 5-2014 - Jul 13, 2025]
- Breakdown by view type
- HTML page views: 14837
- PDF downloads: 4704