Supraglottic Obstruction in an Adult with Inspiratory Arytenoid Cartilage Prolapse
Received: 05-Feb-2014 / Accepted Date: 22-Mar-2014 / Published Date: 29-Mar-2014 DOI: 10.4172/2161-119X.1000165
Abstract
Laryngomalacia (LM) has become the default diagnostic term for any patient who might struggle with stridor and dyspnea secondary to floppy, insufficient, hypotonic, or passively collapsing supraglottal tissue characteristics. Despite the increasing incidence of this diagnosis, the etiology, work-up, and pathophysiology of LM remain poorly understood and controversial to date.
Objectives: The purpose of this paper is to illustrate the history, unique physical examination findings, and multivaried and creative treatments rendered to an adult patient of ours who struggled with profound signs and symptoms of Type 1 LM.
Methods: This is a case study of idiopathic aerodynamic supraglottic collapse that occurred in an otherwise healthy individual who had no prior history of connective tissue disorder, neurodegenerative disorder, or trauma. The patient’s history, examination, treatment, and review of the literature are discussed.
Results: Laryngoscopy is the definitive test to evaluate supraglottic airway collapse. Supraglottoplasty is a safe and effective treatment for adult LM.
Conclusions: The diagnosis of adult aerodynamic supraglottic collapse is an important entity to consider in patients presenting with dyspnea, stridor, or other breathing complaints. This case description illustrates the importance of laryngoscopy when evaluating patients with such signs and symptoms.
Keywords: Laryngomalacia, Supraglottic collapse, Supraglottic obstruction, Stridor
246228Introduction
The most common cause of stridor and dyspnea in infants is LM. The nominal derivation of this congenital condition arises from the Greek word “malakia”, which refers to morbid softening of an organ part. It has been sub-classified into 3 primary variants: Type 1 is characterized by antero-medial prolapse or collapse of the bodies of the arytenoid cartilages over the laryngeal inlet. In Type 2, the antero-posterior dimension of the airway is significantly reduced by abnormally short aryepiglottic folds. The Type 3 form involves abnormal degrees of posterior deflection of the epiglottis during inspiratory aerodynamics, which results in pronounced narrowing of the laryngeal lumen [1]. In the vast majority of these cases, watchful surveillance is indicated as the treatment of choice, owing to the probability of gradual spontaneous resolution of signs and symptoms without medical-surgical management. For those children with intractable LM several surgical approaches have been successfully employed, including lengthening the aryepiglottic folds and de-bulking or anchoring redundant supraglottic mucosa and/or cartilage [2-4].
Acquired airway obstruction in adults caused by excessive, hypotense, hyperactive, or floppy supraglottic tissue has also been described and classified as LM [4,5]. Irritable larynx syndrome and paradoxical vocal fold motion disorders can also result in intermittent occurrences of breathing difficulties. The etiologies in these cases may include vigorous exercise, chronic use of inhaled steroids, and asthmatic bronchospasms with upstream laryngeal sequelae, neurodegenerative illnesses, traumatic brain injury, cerebral vascular accident, connective tissue diseases, laryngeal trauma, and idiopathic laryngeal synkinesis [6-8]. None of these alternative explanations was considered applicable to the diagnosis of airway collapse in the current patient presentation, inasmuch as her symptoms and signs of dyspnea and stridor were clinically significant during restful breathing as well as exertional activities.
The purpose of this paper is to illustrate the history, unique physical examination findings, and multi-varied and creative treatments rendered to an adult patient of ours who struggled with profound signs and symptoms of Type 1 LM.
Case Report
A 61 year old African American female presented to Detroit Receiving Hospital with chief complaints of dyspnea and stridor on exertion, at rest, and during sleep. These symptoms had persisted for several months prior to her visit to our Otolaryngology clinic. She had a past medical history of hypertension, hypercholesterolemia, gastro esophageal reflux disease, diabetes mellitus, and asthma. At the time of our initial examination she was 6 weeks status post C4-C5 and C5-C6 anterior cervical discectomy with fusion for severe neural foraminal stenosis. It should be noted that her breathing difficulties were first diagnosed approximately one year ago prior to this surgical experience. At that time, she was using a CPCP apparatus to improve sleep apnea symptoms. This device was titrated to the minimum level required to eliminate sleep breathing difficulty and snoring. At a pressure of 9 cm H20, disordered breathing ceased, and the tightfitting mask was well tolerated. Additionally, within the same time frame, the patient underwent pulmonary function testing to evaluate symptoms of suspected exercise induced asthma. At baseline, FVC, FVC1, and FEV1/FVC results were all within normal limits, with excellent efforts given by the patient throughout testing. Methacholine challenge result was next performed, followed by post-bronchodilator response testing via a small-volume nebulizer, with 0.083% albuterol solution over 10 minutes. At first dose of methacholine there was a 23% decrease in FEV1. This finding was interpreted by the attending pulmonologist as suggestive underlying asthma. It should be noted that significant improvement in FVC or FEV1 occurred immediately after administration of the bronchodilator, and flow volume loops had normal configuration. Based on all these pulmonary function test results, the patient’s primary care physician concluded that her breathing disturbances during exercise and sleep were likely casually related to moderately severe asthma.
The patient’s immediate post-operative history was uncomplicated and she was discharged home successfully, without the need for supplemental oxygen therapy. Soon after discharge she developed oropharyngeal dysphagia to solids and liquid, in addition to more severe dyspnea, snoring, and sleep apneic symptoms than were evident before such surgery. These exacerbations lead to multiple ER visits over the course of two months for profound dyspnea and intermittent Globus sensations. On each occasion, she was discharged without hospital admission or prescription medication, with the diagnosis of idiopathic breathing complaints likely secondary to asthmatic bronchospasms. As an outpatient, she underwent a polysomnogram. Results revealed mild sleep disordered breathing, with an apnea-hyponea index of 7.8; she was fitted subsequently for a CPAP unit. She was referred to our clinic and pulmonary medicine for follow-up evaluations of her dysphagia and breathing complaints. The pulmonologist reinforced her daily inhaler regimen and recommended that she avoid strenuous physical exercise activities. No other treatments were rendered.
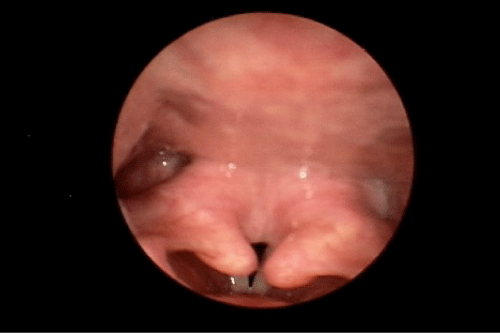
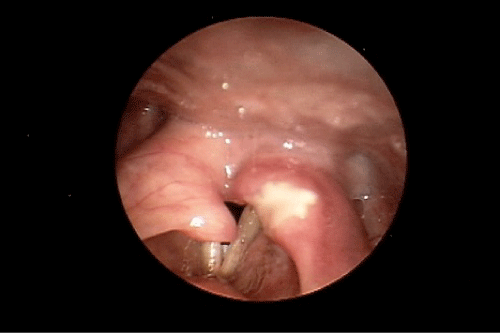
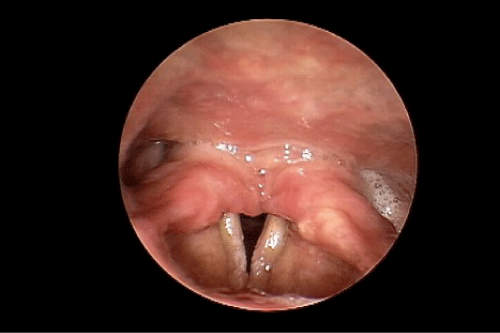
During our examination in the Voice and Swallowing Laboratory the patient appeared well nourished and her speech, voice, and language behaviors were all within normal limits. Stridor was not evident at rest, but deep inspiratory-expiratory exchanges evoked mild-to-moderate bi-phasic noisy breathing sounds. She reported that she had voluntarily altered her food intake to a mechanically soft diet with all liquid preferences. This resulted in a marked improvement in swallowing ability. Anterior rhinoscopy demonstrated a severely deviated left nasal septum. Oral examination results were within normal limits. Videostroboscopy of laryngeal anatomy and physiology revealed an elongated uvula as well as marked inflammation of the arytenoid and corniculate cartilages bilaterally, which aerodynamically prolapsed antero-medially during inspiration. This resulted in near complete obstruction of the laryngeal lumen (Figure 1). The true vocal folds were fully mobile, without identifiable growths or lesions. The false vocal folds, ventricles, epiglottis, and peri-laryngeal boundaries were similarly unremarkable anatomically and physiologically. We elected to refer the patient to the pulmonary service, concurrent with her admission to hospital for airway monitoring and anticipated surgical intervention, Vis a vis a partial uvulectomy and excisional debulking of the left redundant corniculate cartilage and ipsilateral arytenoid body apex. Such surgery was accomplished using the CO2 OmniGuide laser apparatus. On her postoperative visit, the surgical site was healing well and the airway dimensions improved by approximately 50% over the preoperative status (Figure 2). The patient reported significantly less sleep difficulties, dyspnea at rest and upon exertion, and noisy breathing. The prolapsing arytenoid and corniculate cartilages was eliminated on the operated side, accounting for these symptomatic gains. Six weeks later the patient was taken to the operating room for the same surgical procedure on the contralateral side of the larynx. Figure 3 illustrates the successful anatomical and physiological treatment outcome. The patient shortly thereafter experienced near complete resolution of her sleep apnea symptoms and dyspnea at rest and with exercise; there was no perceptible degree of stridor at the 1 week post-operative examination. To date, more than 18 months following these staged surgical procedures, the patient continues to maintain her improvements.
Discussion
LM has become the default diagnostic term for any patient who might struggle with stridor and dyspnea secondary to floppy, insufficient, hypotonic, or passively collapsing supraglottal tissue characteristics. Despite the increasing incidence of this diagnosis, the etiology, work-up, and pathophysiology of LM remain poorly understood and controversial to date. It is a rare diagnosis in older children and adults because in the vast majority of cases the condition is congenital and it either spontaneously improves or is treated surgically early in life. A subset of adolescents and adults occasionally present to ER, pulmonary, and ENT physicians with signs and symptoms that closely mimic those observed in children with congenital LM. In individuals with acquired LM, breathing difficulties are most often experience during exertional activities and delta sleep stages 3 and 4. In cases like this, flexible nasolaryngoscopy during sleep has demonstrated invagination or obstructive prolapse of the arytenoid bodies toward the laryngeal lumen during inspiration [2]. When these signs are observed, they may be considered causally related to associated stridor, intermittent hypoxia, hypercapnia, and hypopnea.
Two alternative LM etiologic theories have been postulated by clinical researchers: neuromuscular and structural. The former explanation suggests the principle feature of supraglottic narrowing or collapse results from underlying muscular incoordination and hypotonia of the components of the unified airway; that ill-coordinated respiratory drive, pulmonary load, and airflow dynamics cause hyperreflexive supraglottal resistance, motion, and medial prolapse of these tissues into the lumen of the larynx [9-12]. Those individuals who possess unusually tall and prominent arytenoid and cuneiform cartilages are considered to be most susceptible to developing LM, whether congenital or acquired. Proponents of this theory believe that the brisk air velocities and negative pressure associated with labored respirations and the Bernoulli Effect induce supraglottic collapse and airway obstruction in this population.
Those who support the structural theory of origin propose that congenital or acquired supraglottal tissue anatomic insufficiency or redundancy produces intermittent or persistent laryngeal inlet narrowing [3,13,14]. Factors such as laryngo-pharyngeal reflux, postnasal drainage, and smoking may exacerbate baseline supraglottic edema and airway compromise, especially during exertional breathing and delta sleep.
Laryngoscopy is the definitive test to appreciate supraglottic airway collapse. This collapse may result as redundant mucosa of the arytenoids cartilages, ventricular folds, or aryepiglottic folds are drawn into the laryngeal lumen during deep inspiration. Patients usually experience stridor during sleep and/or exertion. Supraglottic airway collapse must be differentiated from paradoxical vocal fold movement and vocal fold paralysis, both of which may cause similar airway compromising signs and symptoms.
Our patient was seen by her pulmonary physician for breathing difficulty and pulmonary function tests. She had demonstrated a positive methacholine test challenge result, which led to the presumed diagnosis of asthma, to which her dyspnea and obstructive sleep apnea were attributed. Unfortunately, at that time of testing she did not undergo bronchoscopy or laryngoscopy to rule out other possible etiologies for these conditions. In retrospect, had the pulmonologist placed a stethoscope on the larynx as the patient took deep breaths, evidence of bi-phasic stridor would have been detected as a competing challenge to the sole diagnosis of asthma. In retrospect, the patient’s pulmonologist and primary care physician concluded that in her case of coexisting asthma and LM these two conditions likely acted synergistically to compound her exercise induced dyspnea and sleep apnea; akin to a respiratory mechanical double whammy.
We would suggest that possible upstream airway compromise should always be a component of the differential diagnostic algorithm in patients with clinically significant dyspnea, stridor, and/or sleep apnea. Laryngeal stridor, in particular, requires further work-up via endoscopic examination, preferably with the aid of videostroboscopy for detailed imaging and analysis of the anatomy and bio-mechanics of the glottal and supraglottal tissues. Such examination is often beneficial in the post-treatment period as well, for comparative purposes. Had video endoscopy been performed on outpatient early in her course, accurate diagnosis of LM would have been reached, and appropriate treatments would have been rendered sooner?
The origin of our patient’s LM remains a mystery to us. We have asked ourselves whether her condition was congenital. Was LPR a factor in her presenting signs? Could the condition have been a result of aging hypotonia? Perhaps a combination of anatomical and physiological factors? In cases like ours, treating physicians would be wise to examine the status of all components of the unified airway before concluding the diagnostic process and prescribing treatments. In our clinical experiences, numerous patients have been erroneously treated for asthma with undiagnosed laryngeal pathologies, such as vocal fold paralysis, obstructive tumors, or LM.
Our case study of idiopathic aerodynamic supraglottic collapse occurred in an otherwise healthy individual who had no prior history of connective tissue disorder, neurodegenerative disorder, or history of airway trauma. Referral to our service was made only after several failed treatments for asthma, an otherwise negative cardiopulmonary workup, and following the onset of transient dysphagia. Videostroboscopy revealed the likely cause of her external dyspnea, sleep apnea, and stridor. She suffered from collapse of the redundant arytenoid body mucosa and corniculate cartilages into the airway lumen during inspiration. Her glottis dimension was markedly compromised as a result, with associated noticeable stridor and dyspnea. The impending diagnosis was LM of an acquired form. Surgical management was recommended for definitive airway improvement.
A causal link between supraglottic collapse and obstructive sleep apnea may be more common than we may believe. Comprehensive evaluation is of paramount importance to the differential diagnosis. Surprisingly supraglottic mucosal redundancy in conjunction with adult obstructive sleep apnea/hyponea syndrome has only been described sporadically over the past 15 years [3,4]. Rodriquez et al. [15] reported a case of a 48-year-old female whose obstructive sleep apnea and CPAP/Bi-PAP failure was attributable to severe arytenoid mucosal hyperplasia, her airway symptoms resolved after endoscopic laser excision of the excessive mucosa. Similarly, our patient‘s degree of supraglottic collapse negatively impacted her sleep activity. After supraglottic surgery, she experienced markedly improved sleep patterns and airway relief, as demonstrated by her post-treatment AHI of 5.2.
In cases of adult LM, surgical management may prove necessary for long-term airway improvement, especially during exertional activities and deep sleep. For many years, CO2 laser supraglottoplasty has been shown to be the treatment of choice for infants and children with LM [5]. Supraglottoplasty is a well-established method to relieve the airway obstruction without the need for concurrent tracheostomy. Several surgical approaches have been proposed, including 1) excision of the redundant arytenoid mucosa on the arytenoids, 2) division of the aryepiglottic folds to lengthen the laryngeal inlet in the anteroposterior axis, and 3) resection of the aryepiglottic folds followed by glossoepiglottopexy [6-8]. In order to prevent the complication of postoperative interarytenoid scarring, a mucosal bridge between the resected areas must be preserved. These procedures may be carried out with microscissors and microforceps, or using CO2 laser [16,17]. Our patient’s positive response to surgery was consistent with the outcomes of other clinical researchers who employed the supraglottoplasty technique for their adult patients with LM [18-20]. Use of laser technology during such surgery offers the advantage of hemostasis, blood vessel coagulation with little bleeding, and precision repair [21,22].
Conclusion
Acquired adult LM is very rare. The diagnosis of adult aerodynamic supraglottic collapse is an important entity to consider in anyone presenting with dyspnea, stridor, or other breathing complaints. The importance of conducting laryngoscopy to appraise the upper airway in these patients must be considered. In most cases, supraglottoplasty is a safe and effective treatment for long-term symptomatic relief.
References
- Olney DR, Greinwald JH Jr, Smith RJ, Bauman NM (1999) Laryngomalacia and its treatment. Laryngoscope 109: 1770-1775.
- Richter GT, Thompson DM (2008) The surgical management of laryngomalacia. OtolaryngolClin North Am 41: 837-864, vii.
- Beaty MM, Wilson JS, Smith RJ (1999) Laryngeal motion during exercise. Laryngoscope 109: 136-139.
- Siou GS, Jeannon JP, Stafford FW (2002) Acquired idiopathic laryngomalacia treated by laser aryepiglottoplasty. J LaryngolOtol 116: 733-735.
- Gessler EM, Simko EJ, Greinwald JH Jr (2002) Adult laryngomalacia: an uncommon clinical entity. Am J Otolaryngol 23: 386-389.
- Wiggs WJ Jr, DiNardo LJ (1995) Acquired laryngomalacia: resolution after neurologic recovery. Otolaryngol Head Neck Surg 112: 773-776.
- Murry T, Tabaee A, Owczarzak V, Aviv JE (2006) Respiratory retraining therapy and management of laryngopharyngeal reflux in the treatment of patients with cough and paradoxical vocal fold movement disorder. Ann OtolRhinolLaryngol 115: 754-758.
- Fahey JT, Bryant NJ, Karas D, Goldberg B, Destefano R, et al. (2005) Exercise-induced stridor due to abnormal movement of the arytenoid area: videoendoscopic diagnosis and characterization of the "at risk" group. PediatrPulmonol 39: 51-55.
- Belmont JR, Grundfast K (1984) Congenital laryngeal stridor (laryngomalacia): etiologic factors and associated disorders. Ann OtolRhinolLaryngol 93: 430-437.
- Purser S , Irving L, Marty D (1994) Redundantsupraglottic mucosa in association with obstructive sleep apnea. Laryngoscope 104: 114-116.
- Sataloff RT, Chowdhury F, Joglekar S, Hawkshaw M [2011] Atlas of Endoscopic Laryngeal Surgery. Jaypee Brothers Medical Publishers; New Delhi, India.
- Hui Y, Gaffney R, Crysdale WS (1995) Laser aryepiglottoplasty for the treatment of neurasthenic laryngomalacia in cerebral palsy. Ann OtolRhinolLaryngol 104: 432-436.
- Giannoni C, Sulek M, Friedman EM, Duncan NO 3rd (1998) Gastroesophageal reflux association with laryngomalacia: a prospective study. Int J PediatrOtorhinolaryngol 43: 11-20.
- Solomons NB, Prescott CA (1987) Laryngomalacia. A review and the surgical management for severe cases. Int J PediatrOtorhinolaryngol 13: 31-39.
- Rodriguez Adrados F, Esteban Ortega F, Peña Griñán N (1999) [Massive hyperplasia of the arytenoid mucosa with sleep apnea and stridor. Endoscopic resection by CO2 laser]. ActaOtorrinolaringolEsp 50: 664-666.
- Archer SM (1992) Acquired flaccid larynx. A case report supporting the neurologic theory of laryngomalacia. Arch Otolaryngol Head Neck Surg 118: 654-657.
- Thompson DM (2007) Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. Laryngoscope 117: 1-33.
- Woo P (1992) Acquired laryngomalacia: epiglottis prolapse as a cause of airway obstruction. Ann OtolRhinolLaryngol 101: 314-320.
- Matthews BL, Little JP, Mcguirt WF Jr, Koufman JA (1999) Reflux in infants with laryngomalacia: results of 24-hour double-probe pH monitoring. Otolaryngol Head Neck Surg 120: 860-864.
- Lane RW, Weider DJ, Steinem C, Marin-Padilla M (1984) Laryngomalacia. A review and case report of surgical treatment with resolution of pectusexcavatum. Arch Otolaryngol 110: 546-551.
- Seid AB, Park SM, Kearns MJ, Gugenheim S (1985) Laser division of the aryepiglottic folds for severe laryngomalacia. Int J PediatrOtorhinolaryngol 10: 153-158.
- Jani P, Koltai P, Ochi JW, Bailey CM (1991) Surgical treatment of laryngomalacia. J LaryngolOtol 105: 1040-1045.
Citation: Rutt AL, Dworkin JP, Stern N (2014) Supraglottic Obstruction in an Adult with Inspiratory Arytenoid Cartilage Prolapse. Otolaryngology 4:165. DOI: 10.4172/2161-119X.1000165
Copyright: © 2014 Rutt AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 20993
- [From(publication date): 5-2014 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 16338
- PDF downloads: 4655