Research Article Open Access
759C/T Variants of the Serotonin (5-HT2C) Receptor Gene and Weight Gain in Children and Adolescents in Long-Term Risperidone Treatment
| Nicole del Castillo1*, Bridget Zimmerman M2, Billie Tyler3, Vicki L Ellingrod4 and Chadi Calarge5 | |
| 1Department of Psychiatry, The University of Iowa Hospitals and Clinics, USA | |
| 2Department of Biostatistics, The University of Iowa College of Public Health, USA | |
| 3Department of Psychiatry, University of Iowa, USA | |
| 4Department of Clinical Social and Administrative Sciences, College of Pharmacy, Department of Psychiatry, School of Medicine, University of Michigan, USA | |
| 5Department of Psychiatry, The University of Iowa Carver College of Medicine, Psychiatry Research, USA | |
| Corresponding Author : | Nicole del Castillo Department of Psychiatry The University of Iowa Hospitals and Clinics 200 Hawkins Drive, Iowa City, IA, 52242, USA Tel: 319-356-1616 E-mail: nicole-delcastillo@uiowa.edu |
| Received May 17, 2013; Accepted June 27, 2013; Published June 29, 2013 | |
| Citation: del Castillo N, Bridget Zimmerman M, Tyler B, Ellingrod VL, Calarge C (2013) 759C/T Variants of the Serotonin (5-HT2C) Receptor Gene and Weight Gain in Children and Adolescents in Long-Term Risperidone Treatment. Clin Pharmacol Biopharm 2:110. doi:10.4172/2167-065X.1000110 | |
| Copyright: © 2013 del Castillo N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Great inter-individual variability exists in the susceptibility to gain weight during antipsychotic treatment. Thus, we examined whether the -759C/T variants in the promoter region of the 5HT2C receptor gene were differentially associated with weight gain in children and adolescents in long-term risperidone treatment. Methods: Medically healthy 7 to 17 year-olds, treated with risperidone for ≥ six months, were enrolled. Anthropometric measurements, laboratory tests, and treatment history were obtained upon enrollment and from medical records. The effect of the genotype on the trajectory of age-sex-adjusted weight and body mass index (BMI) z scores before and after the onset of risperidone treatment was investigated.
Results: In 124 subjects (90% males, mean age: 11.8 years) treated with risperidone for a mean of 2.8 years, weight and BMI z scores significantly increased after starting risperidone. This change was similar across the two genotype groups as were changes in several cardiometabolic variables.
Conclusion: In contrast to other reports, the T allele failed to confer protection against excessive weight gain or cardiometabolic abnormalities in this group of children and adolescents chronically treated with risperidone.
| Keywords |
| Antipsychotic; Serotonin gene; Weight gain; Adolescents; Variants; Predictors |
| Introduction |
| Obesity is one of the most serious public health concerns in the 21st century [1]. In particular, childhood obesity tracks into adulthood [2,3], increasing the risk for a variety of chronic conditions and reducing life expectancy [4,5]. Moreover, obesity can result in psychosocial sequelae and interfere with treatment adherence [6]. Thus, the prevention and treatment of obesity are imperative, particularly in children and adolescents. |
| The use of second-generation antipsychotics (SGAs) in the pediatric population has dramatically increased over the last two decades [7,8] despite the potential for significant weight gain which frequently results in obesity [9-11]. This is likely due to several factors including growing evidence for efficacy in a broad range of psychiatric disorders such as schizophrenia, mood disorders, tic disorders, and disruptive behavior disorders [12,13] as well as the need to address increasingly challenging behavior in the least restrictive environments. Therefore, as the use of SGAs grows in this vulnerable population, it is necessary to better understand their effect on weight and cardiometabolic health to optimize their long-term tolerability. |
| SGA-induced weight gain is multifactorial, involving environmental and biological factors [14]. In fact, significant interindividual variability exists in the magnitude of weight gained during SGA treatment [15-17]. In children and adolescents, several factors have been associated with SGA-related weight gain including 1) weight at the onset of SGA treatment, 2) prior and concurrent psychotropic treatments, 3) as well as SGA dose which has been implicated in some, but not all, studies [17-23]. |
| Furthermore, important, albeit small, adult twin studies have estimated the heritability of SGA-induced weight gain at 60 to 80% [24,25], confirming that genetic factors account for, at least, part of the susceptibility to this side effect. In fact, the serotonin 5HT2C receptor gene has been of particular interest due to its association with feeding behavior [26] and the fact that 5HT2C receptor gene knockout mice develop obesity [27]. Moreover, the propensity of antipsychotic medications to cause weight gain appears to be related to their affinity for this receptor [28]. Importantly, a C to T transversion at position -759 (-759C/T) in the promoter region of the 5HT2C receptor gene has been associated with a differential rate of gene transcription as well as obesity and diabetes in the general population [29-33], Furthermore, several studies have implicated these variants specifically in antipsychotic-related weight gain, showing a protective effect of the T allele [28,34-42]. |
| Thus, taking advantage of a unique and well-characterized group of children and adolescents in long-term risperidone treatment, we sought to examine whether the T allele of the 5HT2C receptor gene protected against risperidone-related weight gain. Risperidone is the most commonly prescribed SGA in children and adolescents [43-47]. Notably, it is also thought to be the SGA most susceptible to the effects of various patient- and drug-specific factors regarding its potential to induce weight changes during chronic treatment [48]. This makes investigating predictors of weight gain with this drug particularly promising. |
| Methods |
| Subjects |
| This study has been previously described [20,49]. Briefly, 7 to 17 year-old patients treated with risperidone for six months or more were enrolled, irrespective of their primary psychiatric diagnosis or indication for risperidone. Concurrent treatment at enrollment with additional psychotropics, but not with other antipsychotics, was allowed. Participants with neurological or medical conditions that could confound the metabolic or hormonal assessments were excluded as were pregnant females and those receiving hormonal contraception. |
| Procedures |
| This study was approved by the local Institutional Review Board. Assent was obtained from children ≤ 14 years old and written consent from adolescents and all parents or legal guardians. |
| Race and ethnicity were based on self-report. Using the medical record, the indication for risperidone, the start and stop dates of each psychotropic, and changes in the dosage and formulation were recorded [20]. This documentation was confirmed by a physician and reflected deviations from the prescribed treatments. All dosages of psychostimulants were expressed in methylphenidate-equivalent for amphetamines (x 2) [50]. |
| Upon enrollment, triceps and subscapular skinfold thickness was measured with a Lange skinfold caliper to the nearest 0.1 mm by one of two research dietitians (inter-rater agreement ICC>95%, n=16) [20]. The average of two measurements was used. Height was measured to the nearest 0.1 cm using a stadiometer (Holtain Ltd., UK) and weight was recorded to the nearest 0.1 kg using a digital scale (Scaletronix, Wheaton, IL), while wearing indoor clothes without shoes. In addition, all height and weight measurements were extracted from the medical record. When both height and weight were available, body mass index (BMI, kg/m2) was computed. Importantly, measurements collected during clinical and research encounters falling within a month of each other were highly correlated [mean interval between visits=16 days (sd=9) for height (n=69) and 17 days (sd=9) for weight (n=97)]. The intra-class correlations were all above 0.97 (95% confidence intervals [CI] ranging between 0.96-0.99) for unadjusted height and weight as well as for their sex- and age-adjusted z scores. |
| Pubertal stage was evaluated by a physician and the participants, with parental help when necessary. Interrater agreement between the physician and self-rating was high (weighted kappa=0.81, 95% confidence interval (C.I.)=0.74-0.88). |
| A best-estimate diagnosis, following the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [51], was generated based on a review of the psychiatric record often supplemented by a brief clinical interview, a standardized interview of the parent using the NIMH Diagnostic Interview Schedule for Children (DISC-IV) [52], and the Child Behavior Checklist [53]. |
| A morning blood sample was obtained, after at least a 9-hr overnight fast, to measure glucose (Roche Diagnostics, Indianapolis, IN), total insulin (Diagnostic Products, Los Angeles, CA), total cholesterol, HDL cholesterol (HDL), and triglycerides (Roche Diagnostics, Indianapolis, IN). LDL cholesterol was estimated following Friedewald’s equation [54]. Participants who were not fasting or whose fasting status was missing were excluded from the analyses related to the laboratory measures. |
| DNA was extracted from a whole blood sample using a Purgene kit (Qiagen, Valencia California). After the samples were processed, they underwent spectrophotometry to establish purity and yield and were then frozen at -80°C. Polymerase chain reaction (PCR) and sequencing primers for the -759C/T variants (dbSNP rs3813929) were designed using Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). PCR products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide before Pyrosequencing. Genotyping was done with Pyrosequencing™ Technology [55]. Details regarding this assay are available upon request. |
| Statistical analysis |
| Participants with missing baseline weight (or BMI), i.e., one obtained within one month before the initiation of risperidone, and those exposed to antipsychotics other than risperidone prior to starting risperidone were excluded from the analysis. If, after starting it, a participant discontinued risperidone or received an additional antipsychotic, all subsequent anthropometric measurements were excluded. Also, observations collected before age 2 years were excluded. |
| In order to account for children’s natural growth, age-sexspecific z scores for weight and BMI were calculated [56]. Blood pressure measurements were also converted into age-sex-heightadjusted z scores [9]. Body fat was estimated using skin-fold thickness measurements following Slaughter et al. [57] and the homeostasis model assessment insulin resistance index (HOMA-IR) was estimated as: [insulin (μUI/ml)×glucose (mg/dl)]/405 [58]. |
| The sample was divided based on the presence of the T allele (i.e., T(+) [n=18] versus T(-) [n=106] groups). Differences across the two genotype groups were compared using the Student t-test for continuous variables and Fisher’s exact test for categorical ones. When necessary, analysis of covariance (ANCOVA) was used to adjust for age and sex. |
| In order to explore the genotype effect on the trajectory of weight gain during risperidone treatment, a random coefficient mixed regression model was fitted, having weight (or BMI) z score as the dependent variable and baseline weight (or BMI) z score, the weightadjusted daily dose of methylphenidate (mg/kg/day), genotype group, and genotype group x risperidone treatment status x time 3-way interaction effects as predictor variables. The model included fixed and random effects for intercept and slope (i.e., time), to represent the mean curve and the random variation of each child’s curve from the mean curve, respectively. Since we anticipated that weight gain from risperidone will plateau, time was modeled as a linear and quadratic effect. Consequently, we required a minimum of three weight (or BMI) measurements before or three after risperidone initiation in order for a participant to contribute to the analysis. |
| All the statistical tests were two-tailed, with statistical significance set at α=0.05, and performed using SAS version 9.2 for Windows (SAS Institute Inc, Cary, North Carolina). |
| Results |
| Participants |
| As shown in Table 1, the majority of the participants included in the analysis were males (n=112, 90%) and non-Hispanic Caucasian (n=101, 82%). The T allele carriers were slightly older and more sexually mature than the T(-) genotype group. |
| Table 2 summarizes the psychiatric and treatment characteristics of the sample. Externalizing disorders were prevalent and most participants received risperidone for aggression or irritability (90%). Polypharmacy was common with psychostimulants (73%), selective serotonin reuptake inhibitors (51%), and α2-agonists (31%) being the most concurrently prescribed agents. |
| After adjusting for age and sex, the two genotype groups did not differ on any clinical characteristic except for anxiety disorders being more common among the T(-) group. |
| Measurements at study enrollment |
| Table 3 reports the anthropometric and cardiometabolic measurements of the sample. Over the course of risperidone treatment, weight and BMI z scores increased significantly but this was comparable across the two genotype groups. Again, after controlling for age and sex, none of the cardiometabolic variables differed across the two genotype groups. |
| Trajectory of weight gain |
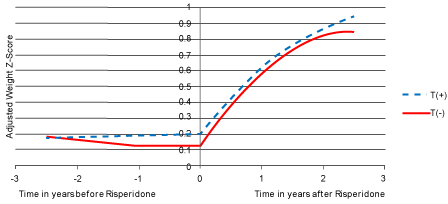
| Using mixed model regression analysis, after controlling for baseline weight z score (β=0.953, p<0.0001) and the weight-adjusted daily dose of methylphenidate (β=-0.173, p<0.0001), there was a significant genotype by risperidone treatment by time 3-way interaction effect (p<0.0001). In fact, the trajectories of change in weight z score before and after risperidone was started were significantly different (p<0.0002). As shown in Figure 1, there was no significant change in weight z score before risperidone was started. In contrast, after the onset of risperidone treatment, weight z score increased significantly. However, the trajectories across the two genotype groups were comparable both before as well as after risperidone was started, for both the linear (p>0.4) as well as the quadratic (p>0.05) effects of time. |
| Similarly, after controlling for baseline BMI z score (β=0.867, p<0.0001) and the weight-adjusted daily dose of methylphenidate (β=- 0.200, p<0.0001) (Figure 2), the genotype by risperidone treatment by time 3-way interaction effect was significant (p<0.0001). Again, this reflected the fact that BMI z score increased significantly after risperidone was started (p<0.0001). Interestingly, the trajectories of BMI z score was different across the two genotype groups before risperidone was started, with the T(+) allele showing a slight increase in BMI z score (p<0.03). However, the trajectories were not different after risperidone was started for either the linear (p>0.8) or the quadratic (p>0.9) effect of time. |
| When the analyses were repeated after restricting the sample to non-Hispanic Caucasians, the findings did not change appreciably. |
| Discussion |
| To our knowledge, this is the first pediatric study to evaluate the potential role of the -759C/T variants in the promoter region of the 5HT2C receptor gene in weight gain following long-term treatment with SGAs. In contrast to our hypothesis and several published studies [17,28,34-42], the T allele was neither associated with less weight gain nor with a reduced risk for cardiometabolic abnormalities. |
| For decades, the role of serotonin in feeding behavior has been of interest with significant advances made delineating the specific pathways involved [59,60]. This has also led to research investigating the role of specific subtypes of serotonin receptors. In particular, the 5HT2C receptor has been studied in regards to food consumption and satiety [60]. For instance, 5HT2C receptor gene knock-out mice overeat, are obese, and exhibit increased serum glucose, leptin, and insulin concentrations [27]. In addition, 5HT2C receptor antagonists have also been found to prevent or delay the onset of satiety [18]. Of interest, several SGAs exhibit a high affinity for the 5HT2C receptor in contrast to first-generation antipsychotics [18,28]. In fact, this affinity appears to be correlated with the propensity of the drugs to cause weight gain [28]. As a result, several studies have explored whether functional variants of the 5HT2C receptor gene are implicated in the risk for antipsychotic-related weight gain. Specifically, the -759C/T variants have been of interest since they are relatively common in the general population. Moreover, the T allele has been associated with increased basal expression of the 5HT2C receptor [33], although this finding has not been consistent [61]. Nonetheless, this focus has made the T allele one of the genetic variants most frequently investigated in antipsychotic-related weight gain [62], with several studies finding it to be protective compared to the C allele [28,34-42]. |
| The protective potential of the T allele has also been recently confirmed in a small trial in children with autistic disorder receiving risperidone for 8 weeks [17]. It is unclear why our findings differed from that study but several reasons could explain the discrepancy. We included a heterogeneous clinical group, in polypharmacy, treated with risperidone for an average of nearly three years. In contrast, the study by Hoekstra et al. [17] was restricted to youth with autistic disorder, treated with risperidone for 8 weeks, with only 25% receiving another psychotropic (namely psychostimulants). Similarly, it is likely that differences in study design explain the discrepancy between studies reporting a protective effect of the T alleles [28,34-42] and those not [63-70]. In fact, factors thought to influence this association include prior antipsychotic treatment status, duration of treatment (< vs. >3 months), the particular drug investigated, race, and sample size [62]. |
| Our participants had undergone treatment with risperidone for nearly three years. Others have shown that acute and chronic antipsychotic-induced weight gain may be linked to different genetic risk factors [71]. Leptin and serotonin both reduce food intake and promote energy expenditure [72]. A variant in the promoter region (-2548A/G) of the leptin gene has been associated with obesity [42]. In antipsychotic-treated patients, Templeman et al. [42] found that the A allele protected against excessive weight gain after 9 months of treatment but not acutely (i.e., after 6 and 12 weeks of treatment). In contrast, carriers of the T allele of the 5HT2C gene, at position -759, gained significantly less weight than those without this allele, following both acute and extended treatment [42]. Interestingly, the -759C/T genotype was significantly associated with pre-treatment plasma leptin levels [42]. In an earlier study, using an overlapping sample, we found that variants of the leptin gene predicted weight gain from risperidone [73]. How the -759C/T variants of the 5-HT2C receptor gene might interact with the -2548A/G variants would be critical to explore since the contribution of any single gene to a complex phenotype, like antipsychotic-related weight gain, is probably limited [74]. |
| There were several limitations to this study. First, unlike most other studies, our sample consisted of a heterogeneous clinical group receiving psychotropic polypharmacy. However, SGAs appear to cause comparable weight gain across most pediatric psychiatric disorders (except eating disorders perhaps) [11,75,76]. In fact, the magnitude of weight gain we found is similar to what others have reported [21]. Some demographic and clinical characteristics differed across the two genotype groups but these may reflect the relatively small sample size of the T allele carriers and the number of statistical comparisons conducted. Nonetheless, we accounted in the analyses for pertinent variables (e.g., age). Another shortcoming is that, except for psychostimulants, we failed to account for treatment with other psychotropics that may alter weight. Thirdly, although risperidone is the most commonly prescribed antipsychotic in children [43-47], it is unclear how our findings might generalize to other antipsychotics. In addition, most anthropometric measurements were extracted from the medical record. However, as we note earlier and as shown by others [77], the measurements collected during clinical encounters were highly correlated with those obtained following standard research procedures. Finally, our sample consisted primarily of non-Hispanic Caucasian males, reflecting the local racial/ethnic composition and national trends of antipsychotic use [12,78]. Thus, future studies should better represent females and participants from a diverse background, while considering that allele frequency might vary across racial/ethnic groups [62,79]. |
| Conclusions |
| In summary, in our sample of children and adolescents in chronic risperidone treatment, the -759C/T variants failed to differentially predict weight gain. The inconsistency in the literature suggests that further research is needed before pharmacogenetics can fulfill its promise to guide clinicians in individualizing antipsychotic treatment. |
| Financial Disclosures |
| Conflict of interest |
| Authors report no biomedical financial interests or potential conflicts of interest. |
| Acknowledgements |
| The authors thank the patients and their families and the staff in the University of Iowa Child and Adolescent Psychiatry clinic and in the Clinical Research Unit. |
| Funding Support |
| This study was funded by a 2005 Young Investigator Award and by the National Institute of Health (RR024979, R21MH080968, and K23MH085005). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. |
References
- Chia DJ, Boston BA (2006) Childhood obesity and the metabolic syndrome. Adv Pediatr 53: 23-53.
- Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH (2007) Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 150: 12-17.
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, et al. (1993) Do obese children become obese adults? A review of the literature. Prev Med 22: 167-177.
- Flegal KM, Graubard BI, Williamson DF, Gail MH (2007) Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298: 2028-2037.
- Malnick SD, Knobler H (2006) The medical complications of obesity. QJM 99: 565-579.
- Toren P, Ratner S, Laor N, Weizman A (2004) Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf 27: 1135-1156.
- Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, et al. (2006) Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr 6: 79-83.
- Patel NC, Crismon ML, Hoagwood K, Johnsrud MT, Rascati KL, et al. (2005) Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 44: 548-556.
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity (2004) Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27: 596-601.
- Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA (2004) A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology 29: 133-145.
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, et al. (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302: 1765-1773.
- Olfson M, Blanco C, Liu L, Moreno C, Laje G (2006) National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 63: 679-685.
- Verdoux H, Tournier M, Bégaud B (2010) Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr Scand 121: 4-10.
- Mulder H, Franke B, van der-Beek van der AA, Arends J, Wilmink FW, et al. (2007) The association between HTR2C gene polymorphisms and the metabolic syndrome in patients with schizophrenia. J Clin Psychopharmacol 27: 338-343.
- Reynolds GP, Kirk SL (2010) Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol Ther 125: 169-179.
- Martinez JA, Parra MD, Santos JL, Moreno-Aliaga MJ, Marti A, et al. (2008) Genotype-dependent response to energy-restricted diets in obese subjects: towards personalized nutrition. Asia Pac J Clin Nutr 17: 119-122.
- Hoekstra PJ, Troost PW, Lahuis BE, Mulder H, Mulder EJ, et al. (2010) Risperidone-induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5-hydroxytryptamine 2C receptor gene. J Child Adolesc Psychopharmacol 20: 473-477.
- Balt SL, Galloway GP, Baggott MJ, Schwartz Z, Mendelson J (2011) Mechanisms and genetics of antipsychotic-associated weight gain. Clin Pharmacol Ther 90: 179-183.
- Safer DJ (2004) A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 24: 429-436.
- Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA (2009) Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 19: 101-109.
- Martin A, Landau J, Leebens P, Ulizio K, Cicchetti D, et al. (2000) Risperidone-associated weight gain in children and adolescents: a retrospective chart review. J Child Adolesc Psychopharmacol 10: 259-268.
- Correll CU, Kane JM (2007) One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol 17: 647-656.
- Calarge CA, Nicol G, Xie D, Zimmerman B (2012) Correlates of weight gain during long-term risperidone treatment in children and adolescents. Child Adolesc Psychiatry Ment Health 6: 21.
- Theisen FM, Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, Wehmeier PM, et al. (2005) Clozapine-induced weight gain: a study in monozygotic twins and same-sex sib pairs. Psychiatr Genet 15: 285-289.
- Gebhardt S, Theisen FM, Haberhausen M, Heinzel-Gutenbrunner M, Wehmeier PM, et al. (2010) Body weight gain induced by atypical antipsychotics: an extension of the monozygotic twin and sib pair study. J Clin Pharm Ther 35: 207-211.
- Simansky KJ (1996) Serotonergic control of the organization of feeding and satiety. Behav Brain Res 73: 37-42.
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, et al. (1995) Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374: 542-546.
- Reynolds GP, Zhang ZJ, Zhang XB (2002) Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 359: 2086-2087.
- Shih JC, Zhu Q, Chen K (1996) Determination of transcription initiation sites and promoter activity of the human 5-HT2A receptor gene. Behav Brain Res 73: 59-62.
- Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K (2000) Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes. Diabetologia 43: 373-376.
- Reynolds GP, Templeman LA, Zhang ZJ (2005) The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 29: 1021-1028.
- Mulder H, Franke B, van der-Beek van der AA, Arends J, Wilmink FW, et al. (2007) The association between HTR2C polymorphisms and obesity in psychiatric patients using antipsychotics: a cross-sectional study. Pharmacogenomics J 7: 318-324.
- Buckland PR, D'Souza U, Maher NA, McGuffin P (1997) The effects of antipsychotic drugs on the mRNA levels of serotonin 5HT2A and 5HT2C receptors. Brain Res Mol Brain Res 48: 45-52.
- De Luca V, Mueller DJ, de Bartolomeis A, Kennedy JL (2007) Association of the HTR2C gene and antipsychotic induced weight gain: a meta-analysis. Int J Neuropsychopharmacol 10: 697-704.
- Godlewska BR, Olajossy-Hilkesberger L, Ciwoniuk M, Olajossy M, Marmurowska-Michał et al. (2009) Olanzapine-induced weight gain is associated with the -759C/T and -697G/C polymorphisms of the HTR2C gene. Pharmacogenomics J 9: 234-241.
- Laika B, Leucht S, Heres S, Schneider H, Steimer W (2010) Pharmacogenetics and olanzapine treatment: CYP1A2*1F and serotonergic polymorphisms influence therapeutic outcome. Pharmacogenomics J 10: 20-29.
- Lane HY, Liu YC, Huang CL, Chang YC, Wu PL, et al. (2006) Risperidone-related weight gain: genetic and nongenetic predictors. J Clin Psychopharmacol 26: 128-134.
- Miller DD, Ellingrod VL, Holman TL, Buckley PF, Arndt S (2005) Clozapine-induced weight gain associated with the 5HT2C receptor -759C/T polymorphism. Am J Med Genet B Neuropsychiatr Genet 133B: 97-100.
- Opgen-Rhein C, Brandl EJ, Müller DJ, Neuhaus AH, Tiwari AK, et al. (2010) Association of HTR2C, but not LEP or INSIG2, genes with antipsychotic-induced weight gain in a German sample. Pharmacogenomics 11: 773-780.
- Ryu S, Cho EY, Park T, Oh S, Jang WS, et al. (2007) -759 C/T polymorphism of 5-HT2C receptor gene and early phase weight gain associated with antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 31: 673-677.
- Sicard MN, Zai CC, Tiwari AK, Souza RP, Meltzer HY, et al. (2010) Polymorphisms of the HTR2C gene and antipsychotic-induced weight gain: an update and meta-analysis. Pharmacogenomics 11: 1561-1571.
- Templeman LA, Reynolds GP, Arranz B, San L (2005) Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics 15: 195-200.
- Doerry UA, Kent L (2003) Prescribing practices of community child and adolescent psychiatrists. The Psychiatrist27: 407-10.
- Findling RL, McNamara NK (2004) Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry 65: 30-44.
- Otasowie J, Duffy R, Freeman J, Hollis C (2010) Antipsychotic prescribing practice among child psychiatrists and community paediatricians. The Psychiatrist 34: 126-9.
- Pappadopulos E, Jensen PS, Schur SB, MacIntyre JC 2nd, Ketner S, et al. (2002) "Real world" atypical antipsychotic prescribing practices in public child and adolescent inpatient settings. Schizophr Bull 28: 111-121.
- Sivaprasad L, Hassan T, Handy S (2006) Survey of Atypical Antipsychotic Medication Use by Child and Adolescent Psychiatrists. Child and Adolescent Mental Health 11: 164-7.
- Gentile S (2009) Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obes Rev 10: 527-42.
- Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA (2010) A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry 71: 338-347.
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, et al. (2007) Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry 46: 1015-1027.
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association; 2000.
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME (2000) NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39: 28-38.
- Achenbach TM, Rescorla L (2001) Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment. Burlington, VT: ASEBA.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
- Ronaghi M (2003) Pyrosequencing for SNP genotyping. Methods Mol Biol 212: 189-195.
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, et al. (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109: 45-60.
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, et al. (1988) Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60: 709-723.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
- Blundell JE (1977) Is there a role for serotonin (5-hydroxytryptamine) in feeding? Int J Obes 1: 15-42.
- Lee MD, Clifton PG (2010) CHAPTER 3.3 - Role of the Serotonergic System in Appetite and Ingestion Control. In: Christian PM, Barry LJ (Eds.), Handbook of Behavioral Neuroscience 331-45.
- Hill MJ, Reynolds GP (2007) 5-HT2C receptor gene polymorphisms associated with antipsychotic drug action alter promoter activity. Brain Res 1149: 14-17.
- Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, et al. (2012) Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry 17: 242-266.
- Basile VS, Masellis M, De Luca V, Meltzer HY, Kennedy JL (2002) 759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet 360: 1790-1791.
- Gregoor JG, van der Weide J, Loovers HM, van Megen HJ, Egberts TC, et al. (2011) Polymorphisms of the LEP, LEPR and HTR2C gene: obesity and BMI change in patients using antipsychotic medication in a naturalistic setting. Pharmacogenomics 12: 919-923.
- Kuzman MR, Medved V, Bozina N, Hotujac L, Sain I, et al. (2008) The influence of 5-HT(2C) and MDR1 genetic polymorphisms on antipsychotic-induced weight gain in female schizophrenic patients. Psychiatry Res 160: 308-315.
- Park YM, Cho JH, Kang SG, Choi JE, Lee SH, et al. (2008) Lack of association between the -759C/T polymorphism of the 5-HT2C receptor gene and olanzapine-induced weight gain among Korean schizophrenic patients. J Clin Pharm Ther 33: 55-60.
- Theisen FM, Hinney A, Brömel T, Heinzel-Gutenbrunner M, Martin M, et al. (2004) Lack of association between the -759C/T polymorphism of the 5-HT2C receptor gene and clozapine-induced weight gain among German schizophrenic individuals. Psychiatr Genet 14: 139-142.
- Tsai SJ, Hong CJ, Yu YW, Lin CH (2002) -759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. The Lancet 360: 1790.
- Ujike H, Nomura A, Morita Y, Morio A, Okahisa Y, et al. (2008) Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry 69: 1416-1422.
- Yevtushenko OO, Cooper SJ, O'Neill R, Doherty JK, Woodside JV, et al. (2008) Influence of 5-HT2C receptor and leptin gene polymorphisms, smoking and drug treatment on metabolic disturbances in patients with schizophrenia. Br J Psychiatry 192: 424-428.
- Müller DJ, Kennedy JL (2006) Genetics of antipsychotic treatment emergent weight gain in schizophrenia. Pharmacogenomics 7: 863-887.
- Ducy P (2011) 5-HT and bone biology. Curr Opin Pharmacol 11: 34-38.
- Calarge CA, Ellingrod VL, Zimmerman B, Acion L, Sivitz WI, et al. (2009) Leptin gene -2548G/A variants predict risperidone-associated weight gain in children and adolescents. Psychiatr Genet 19: 320-327.
- Schellekens H, Clarke G, Jeffery IB, Dinan TG, Cryan JF (2012) Dynamic 5-HT2C receptor editing in a mouse model of obesity. PLoS One 7: e32266.
- Hagman J, Gralla J, Sigel E, Ellert S, Dodge M, et al. (2011) A double-blind, placebo-controlled study of risperidone for the treatment of adolescents and young adults with anorexia nervosa: a pilot study. J Am Acad Child Adolesc Psychiatry 50: 915-924.
- Kafantaris V, Leigh E, Hertz S, Berest A, Schebendach J, et al. (2011) A placebo-controlled pilot study of adjunctive olanzapine for adolescents with anorexia nervosa. J Child Adolesc Psychopharmacol 21: 207-212.
- Arterburn D, Ichikawa L, Ludman EJ, Operskalski B, Linde JA, et al. (2008) Validity of Clinical Body Weight Measures as Substitutes for Missing Data in a Randomized Trial. Obes Res Clin Pract 2: 277-281.
- Comer JS, Olfson M, Mojtabai R (2010) National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996-2007. J Am Acad Child Adolesc Psychiatry 49: 1001-1010.
- Thorisson GA, Smith AV, Krishnan L, Stein LD (2005) The International HapMap Project Web site. Genome Res 15: 1592-1593.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
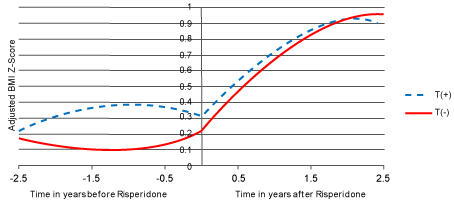 |
| Figure 1 | Figure 2 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14147
- [From(publication date):
October-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9614
- PDF downloads : 4533
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
